Questions
- What does the literature state regarding the methodology, outcome measures, and effectiveness of interventions that provide blood-based laboratory (non-rapid) HIV testing in emergency departments?
Key take-home messages
- There is a large body of evidence examining best practices, outcomes, impact, and other aspects of laboratory (non-rapid) HIV testing in emergency departments (EDs).
- Many studies have identified missed opportunities for HIV testing in the ED (1-5). The literature recognizes a need to streamline the process of obtaining informed consent and ordering HIV tests in ED settings (6) and to provide patients with concurrent HIV testing along with testing for sexually transmitted infections (STIs) (4, 7).
- Methodologies across EDs that conducted laboratory (non-rapid) HIV testing included: testing only individuals that were having other bloodwork conducted (8–11), integrating testing into the current medical flow of the ED (12), screening individuals for HIV testing during triage (9, 10), incorporating processes that made ordering HIV tests akin to other diagnostic tests (8), using the hospital’s electronic medical record system to assist with testing processes (9, 10, 12–14), and collaborating with the hospital’s infectious disease clinic to link individuals with HIV-positive test results to care (13, 15).
- Common outcomes examined included: the proportion of eligible patients offered testing that consented (11, 16, 17), the prevalence of new and previously known HIV diagnoses detected (11, 13, 18, 19), and the proportion of diagnosed individuals linked to care (8, 9, 13, 15).
- Studies found laboratory HIV testing in the ED to be both feasible (8, 16, 20–22) and acceptable (8, 22).
- Studies in the U.S. have demonstrated instances where laboratory blood-based HIV testing in the ED cost less than rapid HIV testing (15, 20, 23). In terms of cost-effectiveness, a 2018 report (24) identified three studies that used laboratory HIV testing in the ED which identified HIV cases above the cost-effectiveness threshold of 0.1% (20, 25, 26). A 2017 Dutch study found that routine, blood-based HIV testing in the ED was not cost-effective, although the study used a low incremental cost effectiveness ratio of EUR 20,000 (27).
The issue and why it’s important
Emergency departments (EDs) are important settings for routine HIV screening because they act as “safety nets” for individuals who are at high risk for HIV and may not have access to primary care services (6, 28). In 2020, recommendations from the Royal College of Emergency Medicine (RCEM) in the UK suggested that routine testing in the ED should be conducted where the local HIV prevalence rate is higher than two per 1,000 population on all adult patients who have blood tests (29). The RCEM suggested ED clinicians should test high risk patients regardless of the local seroprevalence if there is an immediate need (29).
In 2010, New York state passed legislation which required all individuals aged 13 to 64 to be offered an HIV test when receiving care in inpatient, primary care, or ED settings (30). The legislation was updated in 2014 which removed the need for written consent (30). A 2015 retrospective study examined 4,990 remnant blood samples of ED patients in New York City for HIV and found 12 undiagnosed cases which represented 4.8% of all HIV cases detected and a prevalence of 0.2% (1). The study found a higher amount undiagnosed hepatitis C cases among the blood samples with a prevalence of 0.8% (1). The authors stated that the lower prevalence of undiagnosed HIV cases than hepatitis C cases may be attributable to the aforementioned state legislation to test for HIV in settings such as EDs among other factors such as improvement in antiretroviral initiation and increased viral suppression (1).
Various studies have examined missed opportunities for HIV testing in EDs. A 2020 study examined healthcare system encounters and HIV testing among people who use drugs (n=109) across five counties in Kentucky and Ohio (2). Results found that the majority of individuals (69.8%) had presented to the emergency department but were significantly less likely to be tested for HIV than those who were admitted as an inpatient (2). Based on these results the authors suggested that the ED may be an ideal setting to implement HIV testing for people who use drugs (2). A 2020 study in southern Alberta found that among the 393 individuals newly diagnosed with HIV during the study period, 33.1% had an encounter in an ED prior to their diagnosis and about 60% had visited a medical facility in the three years prior to their diagnosis (3). The authors concluded that enhancing testing protocols for those already accessing healthcare services in settings such as the ED can provide substantial benefits to address missed opportunities for an earlier diagnosis (3). Similarly, a 2017 study by Lin et al. suggested that “…future ED screening programs should strive to develop innovative workflows that allow for blood draws for HIV screening only and streamline the processes of obtaining informed consent and ordering tests for all eligible patients” (6).
Another 2020 study examined individuals’ testing patterns for gonorrhea and chlamydia (GC/CT) to determine if they were also tested for HIV at an urban health care system in New York City (4). Results found that although same-day HIV testing rates increased from 2010 to 2015 among both males (59% to 70%) and females (41% to 51%), the ED and inpatient locations were both negatively associated with receiving an HIV test in addition to a GC/CT test (4). Authors concluded that providing risk-based HIV testing to patients with suspected sexually transmitted infections (STIs) in the ED may assist with reducing missed opportunities for HIV testing (4). A 2014 study in a North Carolina ED also found that concurrent HIV and STI testing was low, with HIV testing only occurring among 28.3% of patients tested for syphilis, 3.8% for gonorrhea, and 3.8% for chlamydia over the course of the one-year study (7). A 2020 retrospective study at three EDs in England also found a high prevalence of bloodborne viruses (HIV, hepatitis B, and hepatitis C) with an overall prevalence of 3.3%, of which 67.3% were undiagnosed (31). The undiagnosed prevalence of HIV was estimated to be 0.8% (31). A 2018 Australian study found a large window of opportunity for earlier HIV diagnosis, particularly among patients diagnosed with gonorrhea and syphilis with a median of 15.2 months between hospital admission and HIV diagnosis (5).
A 2020 study retrospectively examined blood samples in an ED in the Bronx, New York to determine how many undiagnosed HIV cases were identified through an untargeted, opt-in screening approach (32). Of the 4,752 blood samples examined that did not have a previous HIV diagnosis, HIV was present in 12 patients, six of which were offered testing during their ED visit, only two (16.7%) of which consented and were diagnosed (32). The authors found that this study reinforced the need to increase the likelihood that those with undiagnosed HIV are offered and consent to HIV testing in the ED (32). Similarly, a 2011 study from Washington, DC examined discarded blood samples from patients who declined HIV testing in an ED and were not known to be HIV positive to determine their prevalence of HIV infection (33). Results found that among 600 discarded blood samples, 12 (2%) were HIV positive (33). This was almost three times higher than the prevalence of HIV infection among the 4,845 patients who accepted HIV testing in the ED, of which 35 (0.7%) tested positive (33). A total of 49% of those who declined testing did so because they believed they were not at risk for HIV (33). As a result, the study authors recommended the development of interventions to decrease the opt-out rate in EDs for routine HIV testing (33). This review explores interventions that have implemented laboratory (non-rapid), blood-based HIV testing in the ED in relation to their methodology, outcomes measures, and effectiveness. Rapid point-of-care HIV testing that can also be used in ED settings is not within the scope of this review.
What we found
ED testing in Canada
From 2010 to 2013, a pilot project called Seek and Treat for Optimal Prevention (STOP) HIV/AIDS Project was implemented in British Columbia to expand HIV testing, diagnosis treatment, and care (34). The pilot offered HIV testing in family practices and acute care as well as targeted testing among high-prevalence populations (34). The pilot was successful in reducing HIV transmission and was implemented provincewide in 2013 with leadership from the BC Centre for Excellence in HIV/AIDS and carried out by the province’s health authorities (35). The Interior Health Authority implemented the program over the next five years which included offering routine HIV testing when requiring bloodwork at local EDs (36).
A 2020 study evaluated the feasibility, acceptability, and effectiveness of implementing routine HIV testing at three Vancouver hospitals in both inpatient and ED settings among eligible patients receiving bloodwork (8). HIV testing was added to routine order sets and public health officials worked with community agencies and acute care physicians to create processes that made ordering HIV tests akin to other diagnostic tests (8). Public health nurses were responsible for following up with all individuals diagnosed with HIV to inform patients of their diagnosis and link them to care (8). Results found that following the implementation of routine testing, the rate of HIV testing increased as much as 11 times compared to the reference period (8). Of 12,996 reviewed patient charts, 5,876 patients were eligible for HIV testing; of which 96.6% agreed to be tested, indicating high acceptability (8). From 2012 to 2016, 151 patients were diagnosed with HIV in the hospitals, 68 (0.1% of all tests) of which were ED outpatients (8). A total of 85.6% of those diagnosed in hospital were linked to care within 30 days which was comparable to linkage rates in the community (8). Based on the results the study authors concluded that routine HIV testing in hospitals was feasible, acceptable, effective, and is a practice that can be normalized and sustained over time (8).
ED testing in the U.S.
Testing for HIV in EDs in the U.S. has increased in recent years. A 2020 report from the Centers Disease Control and Prevention (CDC) stated that the percentage of visits in U.S. EDs that received HIV testing has increased from 0.22% to 0.72% from 2009 to 2017 and a significantly higher percentage of HIV tests were conducted in visits with venipuncture than those without (37).
A 2020 scoping review identified two studies which examined the use of conventional ELISA testing in the ED (38). This included a 2011 retrospective study in a Chicago ED to examine its targeted HIV testing program (39). In just under two years, 1,258 (1.2%) of ED visits resulted in HIV testing, 54 (4.3%) of which were positive for HIV antibody (39). A total of 28 (2.2%) individuals received a new diagnosis, of which 89% were linked to care (39). Authors concluded that the ED HIV testing model was able to successfully identify new patients, provide result notification, and linkage to care (39). The scoping review also included a 2011 study which examined the cumulative effect of an HIV testing program in a midwestern U.S. hospital over a six-year period (40). Of the over 13,000 HIV tests conducted, 0.9% of the results were positive and 12.6% of all individuals tested had at least one repeat test at the ED (40). The number of patients with any previous HIV test increased from 67.7% to 74.4% over the study period (40). The authors stated that testing even a fraction of ED visitors can have a cumulative effect over time on the number of individuals tested as a large portion of patients return to the ED more than once and have more than one opportunity to be tested (40).
A 2016 literature review examining linkage to care rates among individuals in the U.S. diagnosed with HIV in the ED identified three relevant publications (41). Two of these publications examined targeted, opt-out, non-rapid HIV testing as well as patient follow-up at the hospital’s infectious disease clinic (15, 18). This included a suburban ED in North Carolina where over a three-year period, there were 52 (1.9%) patients that tested positive for HIV, of which 0.8% were new diagnoses (18). All previously and newly diagnosed patients were linked to the infectious disease clinic and authors concluded the intervention to be sustainable as it depended on existing available resources (18). Similarly, the second study found 25 new HIV diagnoses in under one year of testing which represented 0.9% of those tested, of which 92% were linked to care (15). The third study implemented routine, opt-out, non-rapid HIV testing for all patients aged 13 to 64 who had their blood drawn in a Houston ED (20). Results found that in under one year there was a low opt-out rate of 0.3% and over 14,000 HIV tests were conducted, of which 80 (0.6%) were new HIV diagnoses (20). The authors found that the intervention was feasible in a busy urban ED, but recognized the need for aggressive follow-up to link patients to care (20).
A 2020 study retrospectively examined a universal HIV screening program from 2010 to 2017 in two southern Texas EDs using fourth generation (antigen/antibody combination) tests (42). All adult ED patients aged 18 to 65 were notified that an HIV test would be performed unless they declined (42). Results found that of all patients tested for HIV over the study period, 0.2% (n=1,795) were new positives (42). The study found a disparity between testing and incidence among African American females who accounted for 16.8% of the tested population, yet represented 20.3% of all HIV positive test results (42). These results indicated a need to focus on vulnerable populations for HIV prevention, testing, and treatment (42). The authors found that populations at high risk for HIV are often cared for in the ED and study results validated the need for HIV testing for this population who may otherwise be missed (42). A 2019 study evaluated a universal, opt-out HIV screening program at two EDs in San Diego (9). The screening process included asking each ED patient during triage if they had HIV or had an HIV test in the last year (9). For all patients who answered “No/Unknown”, a pre-populated HIV testing order appeared in the hospital electronic medical record system if other bloodwork was ordered for the patient (9). Patients were able to opt-out of testing during the blood draw procedure (9). In over 16 months, more than 12,000 individuals were screened for HIV, of which 0.26% (n=33) were newly diagnosed with HIV, 90% of which were successfully linked to care (9). The study also examined the rate of HIV positive individuals who had fallen out of care (9). If a patient was known to be HIV positive during triage and have been out of care for more than a year, they would be contacted by a case manager in an effort to relink them to care (9). The authors stated that newly diagnosed cases may have been lower than expected due to the high number of local HIV testing facilities in San Diego (9). See Figure 1 below for a flow chart of the ED’s HIV testing process (9).
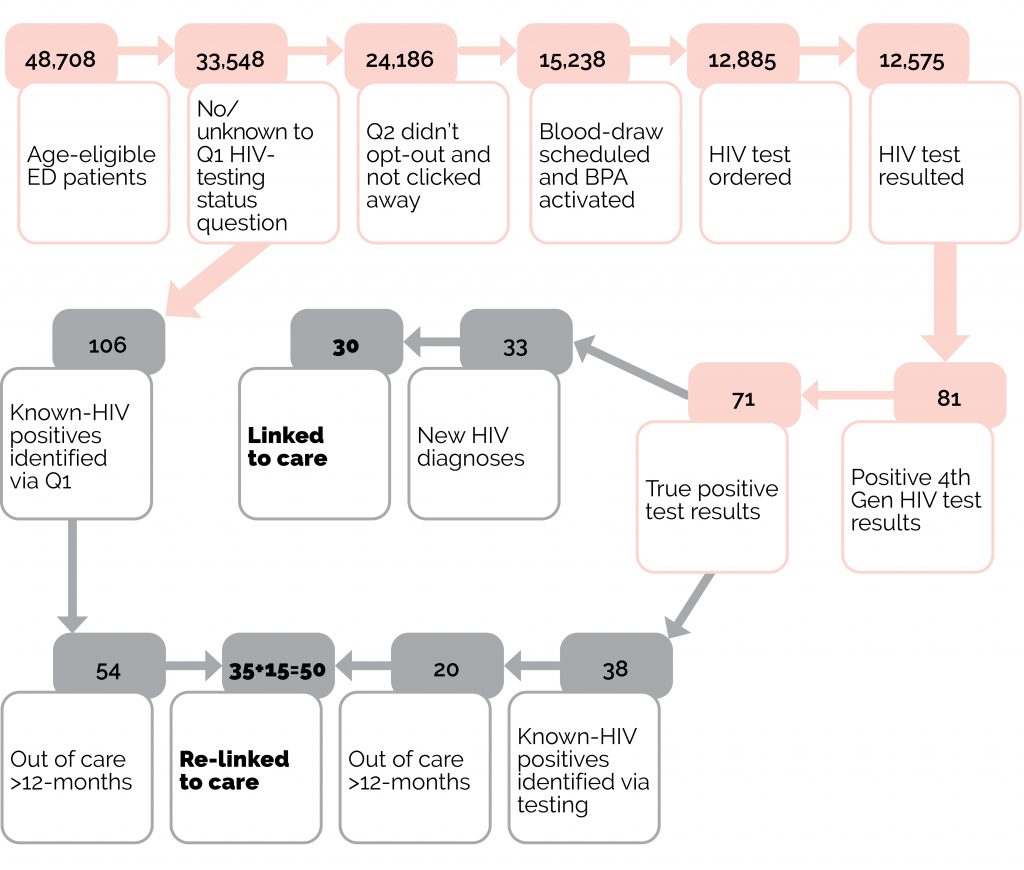
Figure 1: Flow diagram of testing algorithm (pink) and case management flow (grey) from Hoenigl et al. 2019 (9).
Another 2019 study implemented a similar screening process in a Chicago ED with the aim of increasing testing, diagnosis, and linkage to care (13). Beginning in 2015, health providers were prompted to select “order” or “do not order” an HIV antigen/antibody test in the electronic health record for every patient aged 13–64 in the ED who did not have HIV on their problem list or was not tested for HIV in that past year (13). This process was later modified in 2017 to a testing algorithm where an HIV antigen/antibody test was auto-ordered for any patients who met the aforementioned criteria and also had a complete blood count ordered, which led to an increased monthly test rate (13). In over three years of the study, 17.1% of the target population were tested which increased from 2.5% at baseline (13). Of those tested, 164 (0.70%) were confirmed as HIV-positive and 69 (0.29%) were new diagnoses, of which 59 (86%) were linked to care (13).The authors concluded that the program was successful in increasing HIV screening with modifications that minimized additional work for ED staff as well as reduced patient time in the ED (13). The authors indicated the importance of coordination between ED and infectious disease teams as infectious disease staff were responsible for linking patients to care (13).
Various other studies have examined laboratory HIV testing in EDs in the U.S. which included:
- A 2019 study in an ED in Phoenix, Arizona examined the TESTAZ initiative to routinize laboratory-based HIV testing in the ED (10). HIV screening questions were routinized into triage screening through the hospital’s electronic medical record system for all patients receiving bloodwork (10). In the second-year of the initiative nurses were able to order HIV tests during the triage process after screening patients which reduced missed opportunities for testing (10). HIV testing rates increased from 67.2% in the first year to 96.9% in year two (10). A 2014 study of the TESTAZ initiative stated that about a quarter of identified infections were acute which may have been difficult to detect with previous testing methods (43).
- A 2017 study explained the implementation of the HPTN 065 study which aimed to assess the feasibility of a test-and-treat approach for HIV (44). A total of 76 sites in Washington, DC and the Bronx, New York took part in the study, of which 16 were comprised of hospitals (44). The study encouraged hospitals to move towards laboratory-based HIV testing with rapid turnaround to detect HIV cases earlier and maximize efficiency (44). The results found that testing varied greatly across hospitals and the percentage of HIV testing did not significantly change over the course of the three-year study (45).
- A 2016 study examined routine, opt-out HIV testing at three Chicago hospitals from 2012 to 2014 (12). The hospitals were a part of the Frontlines of Communities in the United States (FOCUS) program which had four main principles: 1) organization-wide commitment to routine HIV testing; 2) integrating testing into current workflows; 3) using electronic health records to execute testing and create laboratory orders; and 4) providing staff education on best practices for HIV testing (12). Results found routine screening to be essential for identifying new HIV diagnoses and individuals out of care (12).
- A 2016 study examined routine, opt-out HIV testing among adults aged 18 to 64 in an Alabama ED from 2011 to 2013 (25). Results found over 46,000 HIV tests performed, of which 0.5% were confirmed positive (25). A total of 11.8% were deemed to be acute HIV infections and 76% were linked to care (25). Results found a low testing opt-out rate of 11.6% and was attributed to offering testing routinely, incorporating nurses into the testing workflow early, and offering HIV testing during the initial ED assessment (25).
- A 2016 study examined the implementation of routine, opt-out testing among eligible individuals aged 13 to 64 in an ED in Brooklyn, New York (46). Authors noted that using a continuous quality improvement process by reviewing daily missed opportunities for testing and using these missed opportunities as case studies “…to put a face to the missed tests” decreased opposition to offer and administer tests among health care providers (46). The program also shared the previous day’s HIV testing compliance (number of individuals that agreed to testing who were actually tested) with staff as a way to monitor adherence to testing protocols (46). Results found that such quality improvement processes increased the numbers of HIV testing offers and completed tests, with testing compliance increasing from 77% in 2013 to 98% in 2014 (46).
- A 2015 study examined a program called Routine Universal Screening for HIV (RUSH) in Harris County, Texas EDs which provided HIV testing for adults over 16 years old who had blood drawn or an IV inserted unless they opted out (47). RUSH included service link workers who provided case management, HIV counselling, and linkage to care to diagnosed patients (47). Comparisons were made among patients with an HIV diagnosis who attended the ED before and after the implementation of the RUSH program (47, 48). Results demonstrated a significant improvement in engagement, retention in care, and viral suppression among patients in the RUSH program when compared to the pre-RUSH cohort (47).
- A 2014 study in a North Carolina ED used existing resources to offer laboratory HIV tests to those showing acute HIV symptoms or believed to be at risk, based on the health provider’s discretion (19). Tested patients were given a referral card to the hospital’s walk-in infectious disease clinic where they could obtain their test results a week after their ED appointment (19). Results found an eightfold increase in HIV testing, with 2,436 patients tested for HIV over the study period; 2.3% were positive, of which 0.9% were new diagnoses (19). Among those previously diagnosed, 75% were not in care, of which 63% were successfully linked to an infectious disease clinic appointment (19).
- A 2014 study examined contextual factors that influenced HIV testing rates in three adult EDs in New York City (14). In compliance with New York legislation, all three EDs had an electronic HIV testing order which ensures staff offer an HIV test to every ED patient (14). Results found that 7.9% (n=7,758) of patients agreed to be tested, of which 0.34% (n=26) tested positive for HIV (14). Individuals were significantly more likely to be tested during daytime hours, from July to September, and if other bloodwork had been ordered (14). Additionally, males were more likely to agree to be tested than females, and older adults were less likely to be tested than younger adults (14).
- A 2014 report from the CDC examined the implementation of routine HIV testing programs at a New York health center and a New Orleans ED (26). The initiatives were a part of the FOCUS program and results found the programs to be sustainable, scalable, able to identify new diagnoses, and successful in relinking previously diagnosed individuals to care (26).
ED HIV testing in the UK & Ireland
A 2017 systematic review evaluated studies that examined hospital-based routine HIV testing in high-income countries (49). Two of the included studies focused on using serology HIV testing in an ED in the UK (16, 17). In one of these studies, HIV testing was offered in a London hospital ED to all adults aged 16 to 65 (16). Initially, only oral fluid testing was offered; later, blood testing was included, and nursing staff were incorporated into the intervention, both of which were believed to have a positive impact on the number of patients being offered and accepting a test (16). Outcomes measured included the proportion of eligible patients offered a test, which ranged from 6% to 54% per month, and the proportion of patients that accepted testing, which ranged from 33% to 100% per month (16). The study identified 13 new infections (0.3% of patients tested), all of which were referred to care (16). Authors concluded that routine HIV testing in an inner-city ED is feasible (16). The second study piloted an initiative in another London hospital over a three-month period where all individuals over the age of 16 that had blood taken in the ED were also tested for HIV unless they opted out (17). Among those offered an HIV test, 2,828 (30%) accepted, of which 19 tested positive; eight were new diagnoses (0.28%) (17). A total of 13 individuals who tested positive were linked to care, and eight started taking antiretrovirals (17). The pilot significantly increased HIV testing rates in the ED compared to prior months (17). ED staff found it easier to offer tests to individuals based on policy instead of based on clinical suspicion, and 95% of staff surveyed agreed that routine testing for HIV should permanently continue in the ED (17).
Several other studies examined HIV testing among patients who received bloodwork in the ED. These included:
- A program in Dublin provided all adult patients able to consent and receiving bloodwork with HIV, hepatitis B, and hepatitis C testing unless they opted out (11). The program was initially piloted over a 10-month period from 2014 to 2015 (50), then examined over a 36-month period in a 2020 study (11). This latter study had 63.2% of ED patients receive bloodwork, of which 61.7% accepted HIV testing which resulted in 467 positive cases, 38 (8.1%) of which were new HIV diagnoses (0.1% of all those tested) (11). Of the new diagnoses, 35 (92.1%) were linked to care, of which 29 (96.7%) were virally suppressed one month after data collection (11). Authors concluded that the program was successful in finding new diagnoses and linking individuals to care (11).
- A 2019 study in London found testing for blood-borne viruses such as HIV in the ED to be feasible (21). The study took residual blood samples of adult patients from two EDs that had bloodwork completed as part of routine care from January to June 2015 to test for HIV, hepatitis B, and hepatitis C (21). Results found that the proportions of HIV positive cases found were 1.3% and 2.2% at each hospital ED (21). As the proportions for blood-borne viruses found in the study were higher than in the general population, the results support the notion that higher risk groups may attend urban EDs (21).
- A 2018 study explored the feasibility of expanding testing in a London hospital ED to include HIV, hepatitis B, and hepatitis C testing (51). Results found 24% of ED patients had blood-borne virus testing and another 4.5% had just HIV testing which resulted in 12 new HIV reactive tests (0.1% of those tested) (51). The authors concluded that the opt-out blood borne virus testing program was feasible and effective at identifying new diagnoses (51).
- Four publications described a campaign called “Going Viral” in a London ED (52–55) which was implemented over a nine-moth period (55). The campaign provided all adults receiving a full blood count with HIV, hepatitis B, and hepatitis C testing unless they opted out (55). A total of 6,211 patients tested for at least two of the viruses, and 71 (1.5%) tested positive for HIV, of which 10 were new cases (0.3%) (55). Nine of 16 (56%) patients that required linkage to HIV care presented in late stage of the disease (54). Authors found that that asking patients about recent HIV testing or using an automatic hospital record check to determine if ED patients have been previously tested for HIV may assist with reducing the number of known cases already engaged in care (55, 56). The study concluded that routine screening for blood-borne viruses in the ED may be worthwhile (55).
- A 2016 study examined the acceptability and feasibility of routine, opt-out HIV testing over a three-month period in a London ED among eligible adults aged 18 to 65 (22). Eligible patients were given a leaflet about HIV testing to ensure informed consent (22). Among all the patients offered an HIV test, 65% accepted testing, of which one tested positive (22). The authors concluded that the pilot was acceptable and feasible, and would require leadership, staff training, and additional resources in order to become an effective part of routine ED care (22).
- A 2016 study examined a one-week campaign called TestMeEast which had routine, opt-out HIV testing conducted across six outpatient hospital departments and two EDs in London (57). Banners and leaflets were distributed to patients which stated that “…your blood will be tested for HIV unless you ask us not to” (57). Patients were able to opt out again at the time their blood was drawn (57). Among the 4,317 individuals who had routine blood tests, 2,402 (55.6%) were tested for HIV (57). Results found that eight individuals tested positive for HIV, three of which were new cases who were linked to care (57). The campaign illustrated that hospital staff can be mobilised to do thousands of routine HIV tests during a campaign (57).
- A 2016 study documented routine HIV testing in an inner city ED in the UK over a 36-week period (58). Results found that 64% of ED attendances were tested for HIV, resulting in 172 positive diagnoses, of which 0.3% were new diagnoses (58). A total of 54% of new diagnoses had attended the ED prior to their diagnosis and 23% required an acute admission (58). Authors concluded that the ED is appropriate for opportunistic HIV testing in high prevalence areas (58).
Cost-effectiveness
A literature review conducted by CATIE examined both rapid and laboratory HIV testing in EDs to determine if these methods improved HIV testing outcomes (24). The review stated that there is strong evidence that routine testing in the ED can discover HIV rates above the cost-effectiveness threshold of 0.1%, with sixteen studies illustrating rates above this threshold, of which three examined laboratory based testing (20, 25, 26).
Fourth generation antigen/antibody assays can be completed on blood samples already drawn and therefore do not add additional steps in the workflow of the ED (59). Studies have noted that 15% (60) to 32% (43, 61) of new diagnoses had acute HIV infection which would have not been identified via rapid testing (59). A 2011 study in a U.S. ED found that laboratory-based testing could identify HIV infections earlier after exposure at a lower cost than rapid testing (20). The study found that rapid testing would cost three times as much as conventional testing in their healthcare system, and estimated savings of over USD 98,000 over the course of the study period by using laboratory-based testing instead of rapid testing kits (20).
A 2020 systematic review identified three studies which examined the cost-effectiveness of non-rapid HIV testing in the ED (62). These included:
- A 2017 study which examined the cost-effectiveness of routine, blood-based HIV testing in two Dutch EDs (27). Results found that of 3,223 participants tested, two (0.06%) individuals tested positive which led to an incremental cost-effectiveness ratio (ICER) of EUR 77,050 per quality adjusted life year (27). This was deemed to not be cost-effective based on the relatively low Dutch ICER threshold of EUR 20,000 (27, 62).
- A 2012 study from the U.S. forecasted the costs of both rapid and blood-based (usual care), routine HIV testing in a Veterans Health Administration ED over a seven-year period among a hypothetical cohort (63). The costs of rapid testing included program implementation and disease treatment while the costs of usual care included disease treatment costs only (63). Treatment costs were dependent on the severity of the disease upon diagnosis (63). Results found that the costs were not significantly different between the two types of testing with rapid testing costing USD 1,418,088 and usual care costing USD 1,320,338 when assuming the HIV prevalence was 1% and 80% of patients accepted testing (63).
- A 2011 study measured the societal cost in relation to counselling, testing, and linking patients with new diagnoses to care at five U.S. EDs (15). One of these EDs used non-rapid, targeted HIV testing and had supplemental staff to conduct testing, which resulted in 25 new diagnoses (0.9% of all tests) (15). The estimated cost per patient with a new HIV diagnosis that was linked to care was estimated to be USD 10,200 (15). Another ED with rapid, non-targeted HIV testing that had supplemental staff conduct testing reported 10 new diagnoses (0.8% of all tests) with slightly higher costs than the aforementioned ED, at USD 12,300 per patient linked to care (15). Costs were estimated to be lower in those EDs that used ED providers instead of supplemental staff to conduct testing, had a higher HIV prevalence, and had targeted HIV testing (15). A 2016 study assessed the testing costs of HIV testing among four hospital EDs that participated in the HPTN 065 trial that switched from point-of-care testing to laboratory testing for at least some or all ED patients (23). Results found that costs for laboratory tests were slightly less per patient than rapid point-of-care tests (23). For a reactive result, the cost per patient for laboratory testing was USD 89.29–109.52 while the cost per patient for point-of-care testing was USD 102.03–123.17 (23). The costs for a nonreactive result per patient was USD 17.00–23.83 for laboratory tests and USD 17.64–37.60 for point-of-care tests (23). Study authors estimated that developing an automated process by investing in electronic system interfaces could reduce costs by about 45% for patients with nonreactive results and 20% for patients with reactive results (23).
Factors that may impact local applicability
As many examined studies were conducted in North America, including British Columbia, the results of which may be applicable to the local structure of EDs in Ontario. As stated by Felsen et al. (2020) it is important to note that the studies “…were performed at different points in the HIV epidemic, in ED settings with differing underlying estimates of undiagnosed HIV, evaluated a variety of different screening strategies, differentiated diagnosed and undiagnosed cases of HIV differently, and used different definitions of the population considered eligible for testing” (32). Therefore, the process and outcomes of laboratory ED HIV testing may vary across settings.
What we did
We searched Medline (including Epub Ahead of Print, In-Process & Other Non-Indexed Citations) using a combination of title and abstract terms (Emergency department* or emergency room* or emergency ward*) AND title and abstract term (testing) AND title term (HIV). Searches were conducted on January 21, 2021 and results limited articles published in English from 2010 to present. Reference lists of identified articles were also searched. The searches yielded 365 references from which 63 were included.
Reference list
- Torian LV, Felsen UR, Xia Q, Laraque F, Rude EJ, Rose H, et al. Undiagnosed HIV and HCV infection in a New York City emergency department, 2015. American Journal of Public Health. 2018;108(5):652–8.
- Furukawa NW, Blau EF, Reau Z, Carlson D, Raney ZD, Johnson TK, et al. Missed opportunities for human immunodeficiency virus (HIV) testing during injection drug use-related healthcare encounters among a cohort of persons who inject drugs with HIV diagnosed during an outbreak — Cincinnati/Northern Kentucky, 2017–2018. Clinical Infectious Diseases. 2020;04:04.
- Powell M, Krentz HB, Eagles ME, Gill MJ. Missed opportunities within healthcare for an earlier diagnosis of HIV. International Journal of STD & AIDS. 2020;31(12):1169–77.
- Kapadia SN, Singh HK, Jones S, Merrick S, Vaamonde CM. Missed opportunities for HIV testing of patients tested for sexually transmitted infections at a large urban health care system from 2010 to 2015. Open Forum Infectious Diseases. 2018;5(7):ofy165.
- Mallitt KA, Wilson DP, Jansson J, McDonald A, Wand H, Post JJ. Identifying missed clinical opportunities for the earlier diagnosis of HIV in Australia, a retrospective cohort data linkage study. PLoS ONE. 2018;13(12):e0208323.
- Lin J, Baghikar S, Mauntel-Medici C, Heinert S, Patel D. Patient and system factors related to missed opportunities for screening in an electronic medical record-driven, opt-out HIV screening program in the emergency department. Academic Emergency Medicine. 2017;24(11):135–68.
- Klein PW, Martin IB, Quinlivan EB, Gay CL, Leone PA. Missed opportunities for concurrent HIV-STD testing in an academic emergency department. Public Health Reports. 2014;129 Suppl 1:12–20.
- Gustafson R, Demlow SE, Nathoo A, McKee G, MacDonald LE, Chu T, et al. Routine HIV testing in acute care hospitals: Changing practice to curb a local HIV epidemic in Vancouver, BC. Preventive Medicine. 2020;137:106132.
- Hoenigl M, Mathur K, Blumenthal J, Brennan J, Zuazo M, McCauley M, et al. Universal HIV and birth Cohort HCV screening in San Diego emergency departments. Scientific Reports. 2019;9(1):14479.
- Simon M, McGuire R, Lynch H, Moodey K, Edmonds A, Moore E, et al. The journey toward routinization: Triage nursing and the success of an emergency department-based routine HIV testing program. Journal of Emergency Nursing. 2016;42(2):183–5.
- Grant C, O’Connell S, Lillis D, Moriarty A, Fitzgerald I, Dalby L, et al. Opt-out screening for HIV, hepatitis B and hepatitis C: Observational study of screening acceptance, yield and treatment outcomes. Emergency Medicine Journal. 2020;37(2):102–5.
- Rucker MG, Eavou R, Allgood KL, Sinclair D, Lawal R, Tobin A, et al. Implementing routine HIV screening in three Chicago hospitals: Lessons learned. Public Health Reports. 2016;131 Suppl 1:121–9.
- Sha BE, Kniuksta R, Exner K, Kishen E, Shankaran S, Williams B, et al. Evolution of an electronic health record based-human immunodeficiency virus (HIV) screening program in an urban emergency department for diagnosing acute and chronic HIV infection. Journal of Emergency Medicine. 2019;57(5):732–9.
- Schnall R, Liu N. Timing matters: HIV testing rates in the emergency department. Nursing Research and Practice. 2014;2014:575130.
- Torres GW, Heffelfinger JD, Pollack HA, Barrera SG, Rothman RE. HIV screening programs in U.S. emergency departments: A cross-site comparison of structure, process, and outcomes. Annals of Emergency Medicine. 2011;58(1 Suppl 1):S104–13.
- Rayment M, Rae C, Ghooloo F, Doku E, Hardie J, Finlay S, et al. Routine HIV testing in the emergency department: Tough lessons in sustainability. HIV Medicine. 2013;14 Suppl 3:6–9.
- Bath R, Ahmad K, Orkin C. Routine HIV testing within the emergency department of a major trauma centre: A pilot study. HIV Medicine. 2015;16(5):326–8.
- Martin I, Klein P, Leone P. A collaborative opt-out, non-rapid HIV testing model between an emergency department and infectious disease clinic: 572. Academic Emergency Medicine. 2012;19.
- Hoots BE, Klein PW, Martin IB, Leone PA, Quinlivan EB, Larson JL, et al. Implementation of a collaborative HIV testing model between an emergency department and infectious disease clinic. Journal of Acquired Immune Deficiency Syndromes. 2014;66(3):e67–70.
- Hoxhaj S, Davila JA, Modi P, Kachalia N, Malone K, Ruggerio MC, et al. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Annals of Emergency Medicine. 2011;58(1 Suppl 1):S79–84.
- Cieply L, Simmons R, Ijaz S, Kara E, Rodger A, Rosenberg W, et al. Seroprevalence of HCV, HBV and HIV in two inner-city London emergency departments. Epidemiology & Infection. 2019;147:e145.
- Hempling MC, Zielicka-Hardy A, Ellis JP, Majewska W, Fida G. Routine HIV testing in the emergency department: Feasible and acceptable? International Journal of STD & AIDS. 2016;27(14):1267–74.
- Schackman BR, Eggman AA, Leff JA, Braunlin M, Felsen UR, Fitzpatrick L, et al. Costs of expanded rapid HIV testing in four emergency departments. Public Health Reports. 2016;131 Suppl 1:71–81.
- Challacombe L, Broeckaert L.The routine offer of HIV testing in emergency departments: A review of the evidence. 2018. Canadian AIDS Treatment Information Exchange. Available from: www.catie.ca/en/pif/spring-2018/routine-offer-hiv-testing-emergency-departments-review-evidence Accessed February 1, 2021.
- Galbraith JW, Willig JH, Rodgers JB, Donnelly JP, Westfall AO, Ross-Davis KL, et al. Evolution and escalation of an emergency department routine, opt-out HIV screening and linkage-to-care program. Public Health Reports. 2016;131 Suppl 1:96–106.
- Lin X, Dietz PM, Rodriguez V, Lester D, Hernandez P, Moreno-Walton L, et al. Routine HIV screening in two health-care settings — New York City and New Orleans, 2011–2013. Morbidity and Mortality Weekly Report. 2014;63(25):537.
- Luiken GPM, Joore IK, Taselaar A, Schuit SCE, Geerlings SE, Govers A, et al. Non-targeted HIV screening in emergency departments in the Netherlands. Netherlands Journal of Medicine. 2017;75(9):386–93.
- Rothman RE, Lyons MS, Haukoos JS. Uncovering HIV infection in the emergency department: A broader perspective. Academic Emergency Medicine. 2007;14(7):653–7.
- The Royal College of Emergency Medicine (RCEM). Best practice guideline: HIV testing in the emergency department. 2020. Available from: https://www.rcem.ac.uk/docs/RCEM%20Guidance/RCEM_HIV_Testing_in_the_ED_revised_December_2020.pdf Accessed February 1, 2021.
- City of New York. NYC Health: Reporting diseases & conditions. HIV testing law and CDC recommendations. NYS HIV testing law. 2021. Available from: http://www1.nyc.gov/site/doh/providers/health-topics/aids-hiv-testing-law.page Accessed February 1, 2021.
- Hopkins MJ, Todd S, Beadsworth M, Anderson C, Mohamed Z, Muir D, et al. Consistent high prevalence of undiagnosed blood‐borne virus infection in patients attending large urban emergency departments in England. Journal of Viral Hepatitis. 2020;27(1):88–91.
- Felsen UR, Torian LV, Futterman DC, Stafford S, Xia Q, Allan D, et al. An expanded HIV screening strategy in the Emergency Department fails to identify most patients with undiagnosed infection: Insights from a blinded serosurvey. AIDS Care. 2020;32(2):202–8.
- Czarnogorski M, Brown J, Lee V, Oben J, Kuo I, Stern R, et al. The prevalence of undiagnosed HIV infection in those who decline HIV screening in an urban emergency department. AIDS Research & Treatment. 2011;2011:879065.
- Canadian AIDS Treatment Information Exchange (CATIE). Routine HIV testing in acute care. Vancouver STOP project: Vancouver, British Columbia. 2013. Available from: www.catie.ca/en/pc/stop-hivaids-project Accessed January 27, 2021.
- BC Centre for Excellence in HIV/AIDS (BC-CfE). B.C. launches province-wide expansion of STOP HIV/AIDS program. 2013. Available from: www.bccfe.ca/news/releases/bc-launches-province-wide-expansion-stop-hivaids-program Accessed January 27, 2021.
- Interior Health Authority. HIV tests now part of most emergency department blood work. 2019. The Nelson Daily. Available from: www.thenelsondaily.com/news/hiv-tests-now-part-most-emergency-department-blood-work Accessed January 27, 2021.
- Hoover KW, Huang YA, Tanner ML, Zhu W, Gathua NW, Pitasi MA, et al. HIV testing trends at visits to physician offices, community health centers, and emergency departments — United States, 2009–2017. Morbidity & Mortality Weekly Report. 2020;69(25):776–80.
- Spagnolello O, Gallagher B, Lone N, Ceccarelli G, D’Ettorre G, Reed MJ. The role of targeted HIV screening in the emergency department: A scoping review. Current HIV Research. 2020;22:22.
- Schrantz SJ, Babcock CA, Theodosis C, Brown S, Mercer S, Pillow MT, et al. A targeted, conventional assay, emergency department HIV testing program integrated with existing clinical procedures. Annals of Emergency Medicine. 2011;58(1 Suppl 1):S85–8.e1.
- Hudepohl NJ, Lindsell CJ, Hart KW, Ruffner AH, Trott AT, Fichtenbaum CJ, et al. Effect of an emergency department HIV testing program on the proportion of emergency department patients who have been tested. Annals of Emergency Medicine. 2011;58(1 Suppl 1):S140–4.
- Menon AA, Nganga-Good C, Martis M, Wicken C, Lobner K, Rothman RE, et al. Linkage-to-care methods and rates in U.S. emergency department-based HIV testing programs: A systematic literature review brief report. Academic Emergency Medicine. 2016;23(7):835–42.
- Hill MJ, Cardenas-Turanzas M, Prater S, Campbell JW, McNeese M. Racial and sex disparities in HIV screening outcomes within emergency departments of Harris County, Texas. Journal of the American College of Emergency Physicians. 2020;1(4):476–83.
- Geren KI, Lovecchio F, Knight J, Fromm R, Moore E, Tomlinson C, et al. Identification of acute HIV infection using fourth-generation testing in an opt-out emergency department screening program. Annals of Emergency Medicine. 2014;64(5):537–46.
- Gamble T, Branson B, Donnell D, Hall HI, King G, Cutler B, et al. Design of the HPTN 065 (TLC-Plus) study: A study to evaluate the feasibility of an enhanced test, link-to-care, plus treat approach for HIV prevention in the United States. Clinical Trials. 2017;14(4):322–32.
- Chavez P, Buchacz K, Ethridge S, Branson B, Greene E, Gamble T, et al., editors. Expanding HIV testing in hospital emergency departments and inpatient admissions. Conference on Retroviruses and Opportunistic Infections (CROI) February; 2015.
- Isaac JK, Sanchez TH, Brown EH, Thompson G, Sanchez C, Fils-Aime S, et al. How compliance measures, behavior modification, and continuous quality improvement led to routine HIV screening in an emergency department in Brooklyn, New York. Public Health Reports. 2016;131 Suppl 1:63–70.
- Flash CA, Pasalar S, Hemmige V, Davila JA, Hallmark CJ, McNeese M, et al. Benefits of a routine opt-out HIV testing and linkage to care program for previously diagnosed patients in publicly funded emergency departments in Houston, TX. Journal of Acquired Immune Deficiency Syndromes. 2015;69 Suppl 1:S8–15.
- Centers for Disease Control and Prevention (CDC). Compendium of evidence-based interventions and best practices for HIV prevention LRC chapter — Routine universal screening for HIV (RUSH) program. 2020. Available from: www.cdc.gov/hiv/pdf/research/intervention research/compendium/lrc/cdc-hiv-lrc-routine-universal-screening-for-hiv.pdf Accessed February 1, 2021
- Elgalib A, Fidler S, Sabapathy K. Hospital-based routine HIV testing in high-income countries: A systematic literature review. HIV Medicine. 2018;19(3):195–205.
- O’Connell S, Lillis D, Cotter A, O’Dea S, Tuite H, Fleming C, et al. Opt-out panel testing for HIV, Hepatitis B and Hepatitis C in an urban emergency department: A pilot study. PLoS ONE. 2016;11(3):e0150546.
- Bradshaw D, Rae C, Rayment M, Turner N, Turner R, Pickard G, et al. HIV/HCV/HBV testing in the emergency department: A feasibility and seroprevalence study. HIV Medicine. 2018;19 Suppl 1:52–7.
- Dhairyawan R, O’Connell R, Flanagan S, Wallis E, Orkin C. Linkage to care after routine HIV, hepatitis B & C testing in the emergency department: The ‘Going Viral’ campaign. Sexually Transmitted Infections. 2016;92(7):557.
- Orkin C, Flanagan S, Wallis E, Ireland G, Dhairyawan R, Fox J, et al. Incorporating HIV/hepatitis B virus/hepatitis C virus combined testing into routine blood tests in nine UK emergency departments: The “Going Viral” campaign. HIV Medicine. 2016;17(3):222–30.
- Parry S, Bundle N, Ullah S, Foster GR, Ahmad K, Tong CYW, et al. Implementing routine blood-borne virus testing for HCV, HBV and HIV at a London emergency department — Uncovering the iceberg? Epidemiology & Infection. 2018;146(8):1026–35.
- Bundle N, Balasegaram S, Parry S, Ullah S, Harris RJ, Ahmad K, et al. Seroprevalence and demographic factors associated with hepatitis B, hepatitis C and HIV infection from a hospital emergency department testing programme, London, United Kingdom, 2015 to 2016. Euro Surveillance: European Communicable Disease Bulletin. 2019;24(27).
- Galbraith JW, Franco RA, Donnelly JP, Rodgers JB, Morgan JM, Viles AF, et al. Unrecognized chronic hepatitis C virus infection among baby boomers in the emergency department. Hepatology. 2015;61(3):776–82.
- Bath R, O’Connell R, Lascar M, Ferrand R, Strachan S, Matin N, et al. TestMeEast: A campaign to increase HIV testing in hospitals and to reduce late diagnosis. AIDS Care. 2016;28(5):608–11.
- Hunter L, Larbalestier N, Paparello J, editors. Routine HIV testing in an inner city emergency department — Avoiding missed opportunities for testing. HepHIV Conference Malta; 2017.
- Tan R, Hugli O, Cavassini M, Darling K. Non-targeted HIV testing in the emergency department: Not just how but where. Expert Review of Antiinfective Therapy. 2018;16(12):893–905.
- White DA, Giordano TP, Pasalar S, Jacobson KR, Glick NR, Beverly ES, et al. Acute HIV discovered during routine HIV screening with HIV antigen-antibody combination tests in 9 U.S. emergency departments. Annals of Emergency Medicine. 2018;72(1):29–40. e2.
- Geren K, Moore E, Tomlinson C, Hobohm D, Gardner A, Reardon-Maynard D, et al. Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm — United States, 2011–2013. Morbidity and Mortality Weekly Report. 2013;62(24):489.
- Mwachofi A, Fadul NA, Dortche C, Collins C. Cost-effectiveness of HIV screening in emergency departments: A systematic review. AIDS Care. 2020:1–12.
- Gidwani R, Goetz MB, Kominski G, Asch S, Mattocks K, Samet JH, et al. A budget impact analysis of rapid human immunodeficiency virus screening in veterans administration emergency departments. The Journal of Emergency Medicine. 2012;42(6):719–26.
Suggested citation
Rapid Response Service. Laboratory (non-rapid) HIV testing in the emergency department: Methods, outcomes, and effectiveness. Toronto, ON: The Ontario HIV Treatment Network; February 2021.
Prepared by
Nicole Andruszkiewicz and David Gogolishvili
Photo credit
