Questions
- What health risks and health outcomes are associated with methamphetamine use among gay, bisexual and other men who have sex with men?
- What interventions have proven effective at preventing or reducing the amount and frequency of methamphetamine use among gay, bisexual and other men who have sex with men?
Key take-home messages
- Men who have sex with men who use methamphetamine are more likely to have multiple sexual partners (1–3) and are more likely to engage in condomless anal intercourse (2, 4–7) compared to men who have sex with men who do not use methamphetamine.
- Use of methamphetamine is associated with higher rates of HIV infection (8-13) and syphilis, gonorrhea, and chlamydia (2, 11, 14–16) among men who have sex with men.
- Men who have sex with men who use substances appear to be just as or more likely to use HIV pre-exposure prophylaxis (PrEP) (17), but adherence may not be consistent (17–19).
- Laboratory studies suggest that HIV disease progression and methamphetamine use are correlated (20–22); however, the direct effects of methamphetamine use on HIV pathogenesis are poorly understood (23).
- Several pharmacological, psychosocial and harm-reduction interventions to reduce the harms associated with methamphetamine use were identified; psychosocial interventions (e.g. contingency management, motivational interviewing and cognitive and behavioural therapy) appear to hold the most promise (24).
- No current interventions to prevent the initiation of methamphetamine use among men who have sex with men were identified.
The issue and why it’s important
Methamphetamine, or meth, is a powerful, highly addictive stimulant that can be smoked, snorted, injected, or swallowed (25). Use of methamphetamine is higher among gay, bisexual and other men who have sex with men compared to the general population (1); furthermore, use of methamphetamine among men who have sex with men has increased in the last decade in Canada (26, 27) and in the U.S. (28, 29).
Methamphetamine use is associated with “chemsex”, a colloquial term that describes a particular subset of sexualized drug use among gay, bisexual and other men who have sex with men (30). Other drugs commonly associated with chemsex include gamma-hydroxybutyric acid (GHB), gamma-butyrolactone (GBL), mephedrone (31–35), ketamine, 3,4-methylenedioxy-methamphetamine (MDMA), and cocaine (33). However, crystal meth (a form of methamphetamine) is the most commonly examined illicit substance in studies on chemsex (36). One feature specific to methamphetamine is the kind of “high” it produces (32). Not only does it stimulate the release of dopamine, which enables the reward pathway in the brain to receive high levels of stimulation, it prevents neurons from reabsorbing the extra dopamine; thus, the dopamine remains active for longer (37). Depending on the route of consumption (injected, smoked, snorted, or swallowed) and the amount consumed, the high produced from methamphetamine can last anywhere from four to 12 hours, and can make an individual feel self-confident, euphoric, and energetic (37). Chemsex is growing globally across all age groups of men who have sex with men (38), and appears to be facilitated by technologies such as geolocation-based smartphone applications (so-called dating apps) (38, 39).
Motivations related to substance use and sexual experiences are complex; some individuals perceive substance use as a potential pathway to intimacy and enhanced sexual experiences, a way to access partners and gain entrance into a community, and as a source of empowerment (40). Other research has found that men who have sex with men use methamphetamine to reduce the effects of internalized stigma (41). Indeed, it appears that valued meanings of using methamphetamine among men who have sex with men co-occur with disadvantages and potential risks, a finding supported in the literature (42).
In this review, we examine the health outcomes associated with methamphetamine use among men who have sex with men, and discuss interventions that are effective in preventing and/or reducing methamphetamine use in this population.
What we found
It is well-established in the academic literature that engaging in sexual intercourse while under the influence of psychoactive substances can have negative health outcomes (43). As discussed above, methamphetamine is set apart by the particular high that users experience; this, combined with the neurochemical state of male arousal, creates an overwhelming sexual disinhibition (32). This can contribute to engaging in sexual risk behaviours, such as having multiple sexual partners (1–3) and engaging in condomless anal intercourse (2, 4–7). This section discusses studies that have examined methamphetamine use among men who have sex with men in the context of sexual risk behaviours.
According to a 2022 meta-analysis of eight studies, men who have sex with men who use methamphetamine are nearly four times more likely to have sex with multiple partners compared to men who do not use methamphetamine (1). Among those men who have sex with men who use methamphetamine, analysis based on number of partners showed that the risks of having one to three, four to five, and six or more sexual partners increased by 2.82, 2.98, and 5.89 times, respectively (1).
Other studies (not included in the aforementioned meta-analysis) have demonstrated similar findings. One longitudinal cohort study of gay, bisexual and other men who have sex with men in Vancouver found that crystal methamphetamine use was positively associated with an increase in the number of recent male sex partners in the past six months (2). A cross-sectional study from the UK found that chemsex drug use was strongly associated with reporting eleven or more new sexual partners and participation in group sex (within the past three months) (3).
Condomless anal intercourse is another risk behaviour associated with chemsex. Studies from Canada (2), the U.S. (4, 7), and the UK (5, 6) show that methamphetamine use (and chemsex in general) is associated with condomless anal intercourse among men who have sex with men. Furthermore, one study from the U.S. found that men who have sex with men who primarily inject methamphetamine were more likely to have five or more condomless anal sex partners (44). Another U.S. study found that as methamphetamine use disorder severity increased, rates of condomless anal intercourse with anonymous male partners increased by 44%, and with transactional male partners (i.e. sex in exchange for money, drugs, shelter, food), by 140% (45). However, it is important to note that not all men who have sex with men engaging in chemsex practice unsafe sexual behaviours: one study from the UK found that about one quarter of participants maintained “strict personal rules about condom use with casual partners” despite using multiple illicit drugs (46).
Multiple recently published studies have found an association between chemsex and various infections among men who have sex with men; this includes sexually transmitted infections (STIs) such as syphilis, gonorrhea, and chlamydia (2, 11, 14–16), and sexually transmitted intestinal infection shigellosis (47). A meta-analysis from 2020 found that sexualized drug use was associated with higher odds of bacterial STI and hepatitis C virus diagnoses in men who have sex with men (9).
In a large study of 2,449 sexually active men who have sex with men conducted in three Canadian cities (Montreal, Vancouver and Toronto), 243 (9.9%) of whom reported recent crystal methamphetamine use, the direct association between greater crystal methamphetamine risk and bacterial STI diagnosis (syphilis, gonorrhea and chlamydia) was statistically non-significant (48). However, there was a statistically significant indirect association between crystal methamphetamine use and bacterial STIs via escape motives (defined as the use of substances to escape self-awareness during sex) and negative attitudes toward condoms, which in turn were associated with oral sex and condomless anal sex (48).
It is well-documented that condomless anal intercourse is a high-risk practice for HIV transmission (49), and is the primary mode of HIV transmission among men who have sex with men (50). This is well supported in the literature:
- A meta-analysis from 2015 found that use of methamphetamine is significantly associated with HIV infection among men who have sex with men (8); and
- A more recent meta-analysis (2020) found that sexualized drug use among men who have sex with men predicted higher odds of HIV diagnoses (9).
Several other studies, not included in the two above-mentioned meta-analyses, have also demonstrated that chemsex and/or methamphetamine use is associated with HIV acquisition among men who have sex with men (10–13). One of these studies found that men who have sex with men who were incident users of methamphetamine (i.e. at least one instance of reported use between study baseline and follow-up) and persistent users of methamphetamine (i.e. reported use in the three months prior to baseline and in follow-up) had significantly higher odds of HIV seroconversion (adjusted odds ratio [AOR]=3.95, 95% CI: 1.64–9.47; and AOR=7.11, 95% CI: 4.53–11.17, respectively) (13). Authors also reported that persistent methamphetamine users in this study accounted for more than 35.7% (41 of 115) of HIV seroconversions during the 12-month study period (13).
One safe and effective strategy to reduce the risk of HIV acquisition is by taking pre-exposure prophylaxis (PrEP) (51), though research results on adherence to PrEP among men who have sex with men who use substances appear somewhat mixed. One systematic review from 2022 found that, in general, PrEP adherence among men who have sex with men who use stimulants is poorer (18); this is supported by a recently published study from Los Angeles among men who have sex with men, which found increased odds of inconsistent PrEP engagement when both or either partner reported methamphetamine use (19).
Findings from another systematic review (2022) that examined PrEP care continuum among sexual minority men across 78 studies (in both high- and low-income settings) were mixed (17):
- In 18 out of 30 studies examining associations between substance use and PrEP use, sexual minority men who use substances were more likely to use PrEP (17).
- In 13 of 33 studies that examined associations of substance use with PrEP adherence or persistence, stimulants, chemsex drugs, or club drug use were associated with lower PrEP adherence; however, three of these 33 studies observed associations of stimulant use or chemsex drug use with better PrEP adherence, particularly in the context of recent condomless anal intercourse (17).
- Of the 11 studies on PrEP persistence, five studies found a positive association between stimulant use, cannabis use, and substance use and PrEP persistence (including retention in PrEP care) (17).
This systematic review also found no substantial barriers to accessing PrEP among men who have sex with men (17); one included study even suggests that some men who have sex with men who engage in chemsex are aware of their heightened risk for HIV acquisition and that this may be a key factor influencing their PrEP initiation and adherence (52). This could explain why some research findings report favourable PrEP outcomes among men who have sex with men engaging in drug use:
- While men who have sex with men using crystal methamphetamine are more likely to engage in condomless anal intercourse, they also may be more likely to use PrEP. For example, in one Australian study among HIV-negative or unknown status men with a history of crystal methamphetamine use, 35% reported PrEP use in the previous six months (53);
- Chemsex appears to be significantly associated with correct PrEP use (54); and
- There appears to be no association between missing PrEP doses and engaging in chemsex (55) or stimulant use (56).
A qualitative study explored the biopsychosocial factors related to chemsex experiences and how these influence the use of PrEP (52). Authors found that men who have sex with men used multiple strategies to maintain adherence to PrEP, including restricting the amount or intensity of chemsex participation (e.g. limiting duration of sessions to one night), strategic placement of PrEP (e.g. keeping medication with them/in their bag or car), and external reminders to take PrEP (e.g. setting alarms) (52). Results of an interpretive review support this finding: authors found that men who have sex with men may employ various strategies to balance control and disinhibition when using methamphetamine (42).
On the other hand, we did identify studies that reported negative PrEP outcomes associated with use of drugs among men who have sex with men:
- Engagement in PrEP care (measured in visits) was inconsistent when either one or both individuals in a partnership used methamphetamine (19);
- Lower odds of PrEP adherence were significantly associated with use of stimulants (i.e. methamphetamine, cocaine) before or during condomless anal intercourse (57); and
- Sub-optimal adherence to PrEP in the month after PrEP initiation was significantly associated with stimulant use (i.e. cocaine, methamphetamine, cathinone) (58).
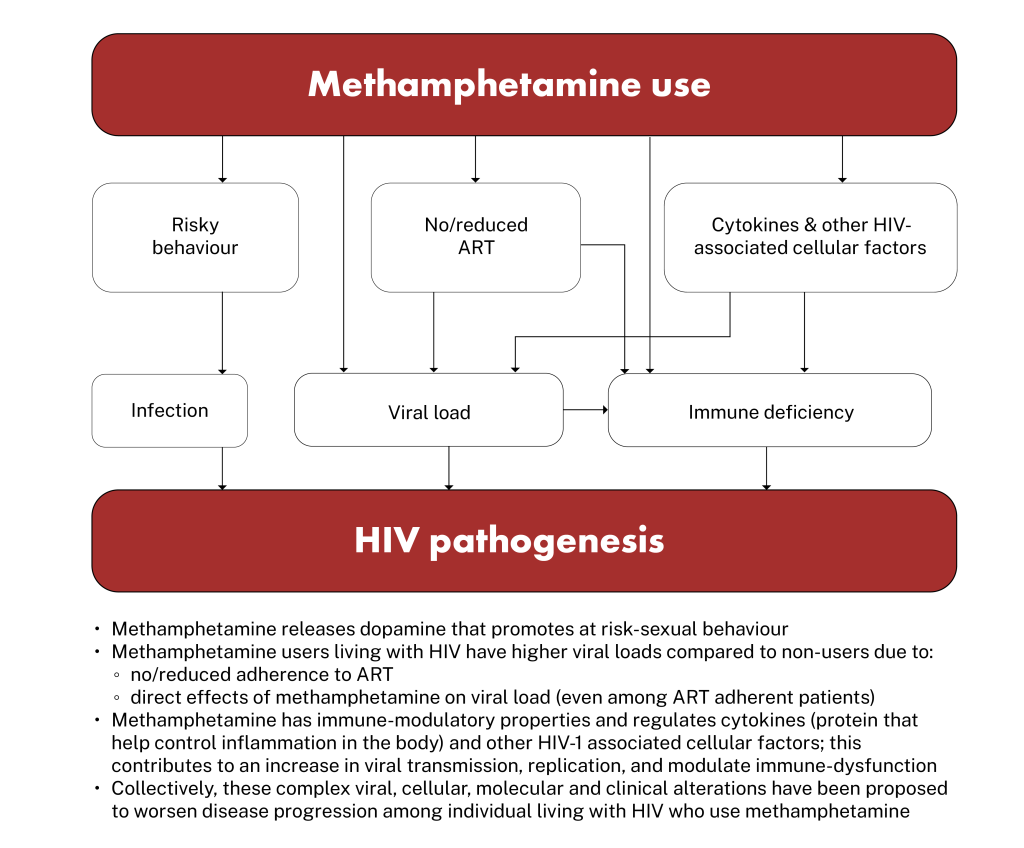
There are several ways that use of methamphetamine can impact HIV pathogenesis, though the mechanisms are not always clear. These pathways are illustrated by a flow diagram in the Figure 1, and explained in more detail below.
Methamphetamine use among men who have sex with men not only impacts risk of HIV acquisition, but also outcomes across the HIV care continuum. In one sample of men who have sex with men living with HIV in Los Angeles who used methamphetamine, authors found that while two-thirds (87 of 129) had an undetectable viral load, only one in four (32 of 129) were at least 90% adherent to antiretroviral therapy (ART) (59). This study also found that participants who reported substance use disorder treatment in the past six months (e.g. overnight stay in a residential alcohol or drug treatment facility, any outpatient substance use disorder treatment, visits with a substance use counsellor) were less likely to be engaged in HIV care, but were nearly three times more likely to be adherent to ART (59). Another study found that the mortality rate for HIV-positive methamphetamine-dependent men who have sex with men (defined as those who sought outpatient behavioural treatment for methamphetamine dependence) in Los Angeles was 1.5 times that for men without HIV, and concomitant HIV infection and tobacco use were associated with five-fold increases in mortality among methamphetamine-dependent men who have sex with men (60).
Figure 1: Methamphetamine and HIV pathogenesis. Adapted from Passaro et al. 2015 (23)
Other studies have found that methamphetamine use impacts viral suppression of HIV. In one study among people living with HIV, where 78% of the sample was comprised of sexual minority men, increasing odds of viral suppression over time were observed among users who decreased use of stimulants (61). In another study, viral suppression was observed at significantly fewer visits among men using methamphetamine (62); an additional study found that methamphetamine was associated with nearly two-fold increased odds of unsuppressed viremia, independent of adherence or other sociodemographic factors (63).
Laboratory studies have also suggested that HIV disease progression and methamphetamine use are correlated (20–22); however, the direct effects of methamphetamine use on HIV infection and HIV disease progression are still poorly understood (23). Nonetheless, research continues to explore physiological harms associated with use of methamphetamine in people living with HIV (20). Researchers hypothesize that methamphetamine use and HIV infection together increase systemic inflammatory processes; this, in turn, increases the risk of morbidity and mortality (60, 64, 65).
HIV and methamphetamine use are both independently associated with neuropathological changes in the brain; this may result in neuropsychiatric disturbances and cognitive disorders (66). Combined, the effects of HIV and methamphetamine can contribute to the clinical complexity of HIV-associated neurocognitive disorders (HAND) by synergistically causing dysfunction of neurovascular units and damage to the blood-brain barrier (67). This is characterized by worsening neurocognitive impairments in executive functions (67), learning and memory (68–70), and motor skills (68–70), resulting in impaired daily functioning (71). However, the exact mechanisms involved with these neurological deficits are not fully understood (72).
Other possible physiological harms associated with methamphetamine use among people living with HIV include:
- Higher rates of frailty, defined as increased vulnerability to multisystem damage (71); and
- Greater likelihood of being classified as a problematic sleeper, which was associated with several adverse outcomes including unemployment, decreased physical and mental quality of life, decreased independence in daily activities, and clinician-rated functional disability (73).
Studies examining physiological disturbances specifically among men who have sex with men have found that:
- Regardless of HIV status, methamphetamine users had greater odds of abnormal thyroid stimulating hormone levels and significantly higher levels of the hormone triiodothyronine (74), which plays an important role in the body’s control of metabolism;
- Regardless of HIV status, methamphetamine use was associated with increased rectal mucosal inflammatory cytokines (64), which has been shown to influence HIV transmission and replication (75);
- Despite being virally suppressed, recent methamphetamine use and greater self-reported substance use severity among men who have sex with men living with HIV was independently associated with higher soluble CD14 levels, a marker of monocyte activation that independently predicts accelerated HIV disease progression (76);
- Methamphetamine use in men who have sex with men living with HIV was an important driver of microbiome variation in the gut, which can cause intestinal dysbiosis (microbial imbalance in the gut) (77); similar results have been found in HIV-negative men who have sex with men who use methamphetamine (78);
- HIV persistence in immune cells and ongoing methamphetamine use in men who have sex with men is predictive of dysregulated catecholamine (e.g. dopamine) synthesis (79), a neurotransmitter that mediates function of the central nervous system;
- Methamphetamine (and cocaine) use were risk factors during a protracted outbreak of serogroup C meningococcal disease among men who have sex with men (80).
Sexualized drug use has also been found to impact the mental health of men who have sex with men. A systematic review published in 2021 concluded that men who have sex with men who practiced sexualized drug use (including chemsex and slamsex, which is the administration of chemsex drugs intravenously) were more likely to experience depression, anxiety, or a substance dependence disorder (81). Data from the Ontario HIV Treatment Network Cohort Study (OCS) demonstrated that among men who have sex with men living with HIV, crystal methamphetamine use is higher in men with greater depressive symptomology (82). Other research has explored these associations, with one study finding that men who have sex with men who use methamphetamine and are diagnosed with higher-intensity depressive symptoms engage in sexual risk-taking behaviours (such as condomless anal intercourse or sex work) at a higher rate with anonymous partners (83).
A 2022 systematic review examining the relationship between chemsex and the development of psychotic symptoms and disorders found that slamsex, polydrug use and smoked methamphetamine posed up to a three-fold increased risk of psychosis (84). A 2023 review found mixed results regarding the relationship between chemsex and suicidality outcomes, though authors note suicidality might be an issue of concern among chemsex users in particular (85).
One systematic review published in 2019 found limited evidence on the impacts of chemsex on psycho-social well-being and functioning (34). However, we did find some recently-published research describing problematic impacts and harms of chemsex on relationships and social-well being. A study conducted among men who have sex with men living with HIV in the UK, Spain, Greece, and Italy found that of the participants who engaged in chemsex (n=382), negative life impacts were reported in the following areas: work (n=96; 25.1%), friends or family (n=93; 24.3%), and intimate relationships (n=108; 28.3%) (86).
Methamphetamine use among transgender individuals
Generally, research on sexualized drug use among transgender individuals is scant (87, 88). One systematic review from 2021 investigated the associations between chemsex and health outcomes among lesbian, gay, bisexual and trans individuals, and identified 75 studies for inclusion; 71 were among men who have sex with men and four were among transgender individuals (87). Of these four studies, only two were conducted in high-income settings (89, 90).
One of these two studies examined sexual risk factors among trans female youth in San Francisco and found that use of crack or cocaine was significantly associated with an increase in condomless receptive anal intercourse (90). The other study, also conducted in San Francisco, found that transwomen who reported any methamphetamine use, and methamphetamine use before or during anal intercourse, had greater odds of testing positive for HIV (89).
Interventions to prevent and/or reduce methamphetamine use among men who have sex with men
We could not identify any interventions that sought to prevent the use of methamphetamine among men who have sex with men. In a previous Rapid Response (2020) that explored best practices for communicating sexual and drug-related harms to prevent uptake of methamphetamine (91), we found one media campaign aimed at discouraging use or uptake of methamphetamine among men who have sex with men with mixed results (91, 92). Specifically, a study of three public health campaigns in New York City in 2004 found that the gay and bisexual community’s reaction to the anti-crystal methamphetamine campaigns were mixed (92):
- Men of colour reported having discussions with partners and friends about their crystal methamphetamine use;
- White men, HIV-negative men, and men not currently using crystal methamphetamine responded more positively to the campaigns than their counterparts; and
- Men who reported recent use of crystal methamphetamine with sex were more likely to report that the campaigns triggered urges to use (92).
Recent qualitative research among key informants working in a range of relevant health and community sectors regarding the stigmatization of crystal methamphetamine use and sexual practices among men who have sex with men found that “…mass media anti-drug campaigns were seen to be a significant generator of stigma with irrelevant and patronizing messages that lacked useful information” (41). Authors concluded that messaging regarding sexuality, HIV, and drug use in social structures and institutions (e.g. media campaigns) should consider the effects of stigma (41).
The vast majority of literature we identified was focused on reducing methamphetamine use among men who have sex with men (rather than preventing methamphetamine initiation). A systematic review from 2019 by Knight et al. sought to identify effective intervention strategies that addressed harms among men who have sex with men who use methamphetamine (24). Authors identified 26 unique interventions (all from high-income settings), and categorized them as follows: pharmacological (n=5), psychosocial (n=20), and harm reduction (n=1) (24).
Pharmacological interventions
Knight et al. (2019) concluded that pharmacological interventions demonstrated limited efficacy (24). This is supported by a 2020 systematic review from Siefried et al., which sought to provide a summary of the current status of research on pharmacological treatment of amphetamine/methamphetamine dependence among individuals in the general population (93). While Siefried et al. (2020) concluded that no pharmacotherapy yielded convincing results for the treatment of amphetamine/methamphetamine dependence, two studies were cited that examined mirtazapine (an antidepressant) to reduce methamphetamine use among men who have sex with men; both studies found reductions in both methamphetamine use and high-risk sexual behaviours (93–95). The first study was a 12-week double-blind randomized controlled trial of mirtazapine versus placebo conducted between 2007–2010 among men who have sex with men actively using methamphetamine in San Francisco (94). Authors found that the addition of mirtazapine to substance use counseling decreased methamphetamine use and resulted in decreased sexual risk behaviours despite low adherence to the medication (94). This study was replicated during 2013–2017 and had similar results: adding mirtazapine to substance use counseling reduced methamphetamine use and HIV risk behaviours among men who have sex with men despite suboptimal medication adherence (95).
Knight et al. (24) and Siefried et al. (93) also examined the impact of bupropion (an antidepressant) on amphetamine/methamphetamine use, and found no significant difference between placebo and intervention groups. However, a 2023 meta-analysis by Bakouni et al. examining placebo-controlled randomized trials to determine the efficacy of bupropion for the treatment of individuals with amphetamine-type stimulant use disorder had different findings (96). Across eight randomized controlled trials, authors found that bupropion showed a significant but modest reduction in amphetamine use and amphetamine cravings (96). However, it was also noted that the clinical relevance of the small effect should be verified (96).
Psychosocial interventions
Knight et al. (2019) found that psychosocial interventions held the most promise; this category mainly included studies examining contingency management (a behavioural intervention in which material incentives are delivered contingent on biological confirmation of drug abstinence) (97), with a few examining motivational interviewing and cognitive and behavioural therapy (24). Some contingency management interventions resulted in reduced methamphetamine use and/or reduced sexual risk behaviours among men who have sex with men (24). Of note, we also identified a 2020 systematic review by Brown et al. that focused specifically on contingency management as an intervention for methamphetamine use among individuals in the general population (97). Brown et al. included nine interventions among men who have sex with men in their review, which included four studies (98-101) published after the Knight et al. review (97). All four studies demonstrated the utility of contingency management programs (98-101). Brown et al. (2020) concluded that outpatient programs offering treatment for methamphetamine use disorder should prioritize adoption and implementation of contingency management interventions based on the positive results (97).
Knight et al. (2019) included one study by Parsons et al. (2014) on motivational interviewing with promising results (24, 102).Young men who have sex with men were randomized into four sessions of motivational interviewing or “content-matched” education; at 52-week follow-up, participants in both conditions reported a reduction in methamphetamine use, while participants in the motivational interviewing condition were less likely to engage in condomless anal intercourse (24, 102). In 2018, Parsons et al. published the findings of another randomized controlled trial that showed efficacy for a combined motivational interviewing and cognitive behavioural therapy intervention for men who have sex with men living with HIV (103). Authors found that days of methamphetamine use decreased by more than 60%, days of medication adherence increased by two days for each subsequent 14-day recall period, and percentage of participants reporting condomless anal sex decreased from 82% to 50% (103).
Another intervention included by Knight et al. was a behavioural activation intervention (a treatment for depression that aims to provide patients with the ability to re-engage in daily life) combined with HIV risk-reduction counseling (24, 104). At follow-up, participants reported a decrease in methamphetamine use and condomless anal intercourse (24, 104). However, the sample size was small (n=16), and authors suggested conducting a randomized controlled trial to determine efficacy (104). In 2019, authors published a study describing results of the randomized controlled trial, which demonstrated that individuals who received the intervention (i.e. behavioural activation and sexual risk reduction counseling) engaged in less condomless anal intercourse with men living with HIV or who had an unknown status (105). Similar results were observed for men engaging in condomless anal intercourse while using crystal methamphetamine (105). Finally, participants in the intervention reported a significantly longer period of continuous days of abstinence from crystal methamphetamine when compared to those in the control group (105). Knight et al. (2019) identified one text-message intervention from 2012 by Reback et al., classified as a psychosocial intervention, which provided interactive and passive social support and health education text messages to men who have sex with men (24, 106). Reback et al. (2012) observed significant decreases in the frequency of methamphetamine use and condomless anal intercourse while using methamphetamine (24, 106). This 2012 study was also included in a 2020 systematic review by Ameri et al. examining the effects of mobile phone-based interventions on methamphetamine use and high-risk sexual behaviours in men who have sex with men (107). Five studies, all authored by Reback et al., published between 2012-2019, were included in the Ameri et al. systematic review (107). The review concluded that the use of text messaging (interactive text conversations with Peer Health Educators, automated theory-based messages, and self-monitoring text-message assessments) significantly decreased the rates of methamphetamine use, condomless anal intercourse, and HIV transmission among men who have sex with men (107).
Harm reduction interventions
The single harm-reduction intervention identified by Knight et al. was community-based, and included three main harm reduction strategies:
- transitioning to less potent modes of methamphetamine administration (e.g. injecting to smoking, smoking to snorting);
- promoting self-care strategies while using methamphetamine; and
- delivering education about safer injection practices with linkage to needle exchanges and access to sterile syringes (24, 108).
Participants of this intervention reported reductions in methamphetamine use, erectile dysfunction medication use in combination with other substances (i.e., while “partying”), and sexual risk-taking behaviour while using methamphetamine (108).
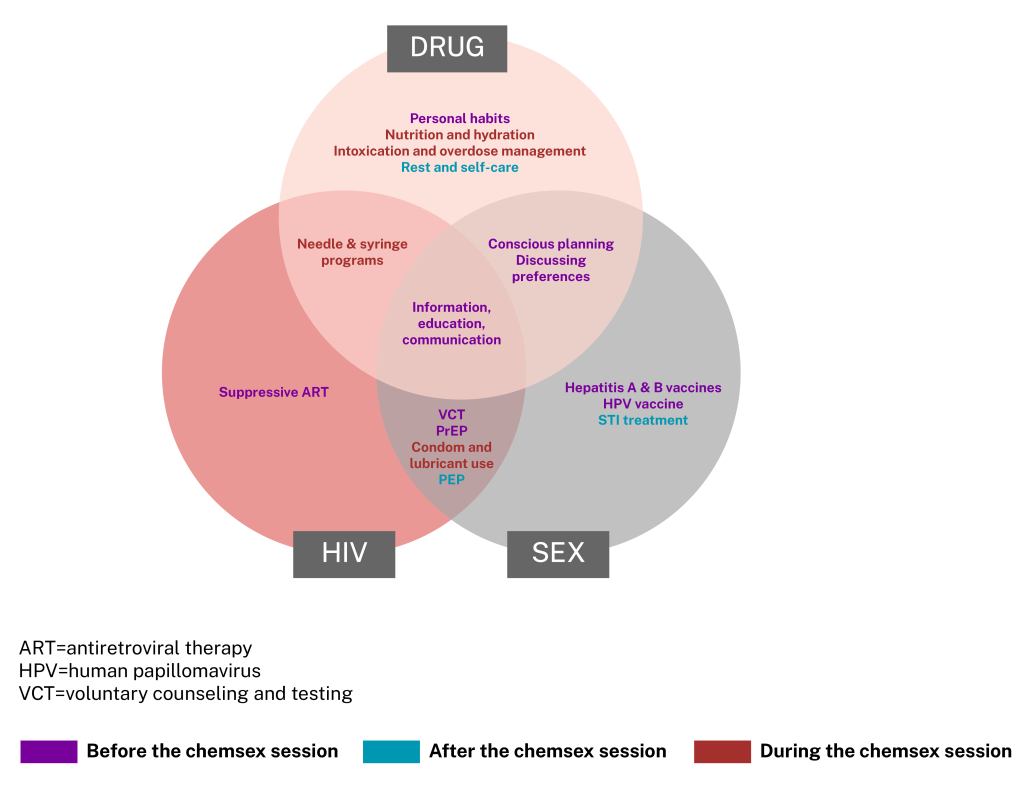
A 2022 review in The Lancet suggested that because methamphetamine-associated harms can be grouped into three distinct types (HIV-, drug-, and sex-related), strategies to reduce these harms can be grouped accordingly, and can be deployed before, during, or after the chemsex session (33). This is illustrated in Figure 2. This review calls to develop tailored harm-reduction models that can accommodate men who have sex with men who engage in chemsex in various ways and with varied effects (33).
Figure 2: Proposed scheme of harm reduction strategies for chemsex. Adapted from Strong et al. 2022 (33)
Harm Reduction International (an international, not-for-profit non-governmental organization based in the UK) also provides recommendations (39) regarding harm reduction for men who have sex with men engaging in chemsex that complements Figure 2. These include:
- meaningful involvement of the community at every stage (e.g. design, implementation, evaluation)
- investment in and scale up of community-led harm reduction services for sexualized drug use
- integrated service delivery covering harm reduction, sexual health, and mental health services with a focus on men who have sex with men
- an online presence, as digital technologies have a central role in the chemsex scene
- a one-stop-shop for all harm reduction commodities (e.g. syringes, pipes, condoms, lubricants, PrEP, PEP)
- easy access to HIV, hepatitis C, and other STI testing, counseling, and treatment
- appropriate knowledge of the language used in the community and the sexual practices that can occur
- appropriate knowledge of the drugs used in the local scene and the possible interactions
- meeting people where they are, and treating them with respect and dignity
- educating professionals working on the ground, and provide trainings to increase awareness of chemsex (39).
Factors that may impact local applicability
Exposure to methamphetamine—often termed methamphetamine “use” or “abuse” in the literature—was a self-reported variable in the majority of included studies. Frequency, duration, amount, and form of methamphetamine (e.g. smoked, snorted, injected) were not consistently described, defined, or analysed across studies. Many studies did not include biomarkers of recent methamphetamine or substance use or PrEP adherence, introducing a possibility of information bias. Additionally, while studies mainly used condomless anal intercourse and number of sexual partners among men who have sex with men as variables to measure risk behaviour, there are likely other aspects influenced by methamphetamine use that have not been explored.
What we did
We searched PsycInfo and Medline (including Ovid MEDLINE and Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations) using text terms (men who have sex or MSM or gay* or GBMSM) AND (chemsex or chem sex or crystal meth or methamphetamine* or PNP or meth or slam* or Party adj2 play). Searches were conducted on October 10, 2023 and results limited to articles published from 2015 to present in English. Only studies conducted in high-income countries were included. Reference lists of identified articles were also searched. Google (grey literature) searches using different combinations of these terms were also conducted. The searches yielded 745 references from which 108 were included.
Reference list
- Moradi S, Moradi Y, Rahmani K, Nouri B, Moradi G. The association between methamphetamine use and number of sexual partners in men who have sex with men: A systematic review and meta-analysis. Substance Abuse Treatment, Prevention, and Policy. 2022;17.
- Colyer SP, Moore DM, Cui Z, Zhu J, Armstrong HL, Taylor M, et al. Crystal methamphetamine use and initiation among gay, bisexual, and other men who have sex with men living with HIV in a treatment as prevention environment. Substance Use & Misuse. 2020;55(14):2428–37.
- Sewell J, Miltz A, Lampe FC, Cambiano V, Speakman A, Phillips AN, et al. Poly drug use, chemsex drug use, and associations with sexual risk behaviour in HIV-negative men who have sex with men attending sexual health clinics. International Journal of Drug Policy. 2017;43:33–43.
- Brown RE, Turner C, Hern J, Santos GM. Partner-level substance use associated with increased sexual risk behaviors among men who have sex with men in San Francisco, CA. Drug & Alcohol Dependence. 2017;176:176–80.
- Curtis TJ, Rodger AJ, Burns F, Nardone A, Copas A, Wayal S. Patterns of sexualised recreational drug use and its association with risk behaviours and sexual health outcomes in men who have sex with men in London, UK: A comparison of cross-sectional studies conducted in 2013 and 2016. Sexually Transmitted Infections. 2020;96(3):197–203.
- Pufall EL, Kall M, Shahmanesh M, Nardone A, Gilson R, Delpech V, et al. Sexualized drug use (‘chemsex’) and high-risk sexual behaviours in HIV-positive men who have sex with men. HIV Medicine. 2018;19(4):261–70.
- Ivey K, Bernstein KT, Kirkcaldy RD, Kissinger P, Edwards O, Abara WE. Chemsex drug use among a national sample of sexually active men who have sex with men—American Men’s Internet Survey, 2017–2020. Substance Use & Misuse. 2023;58(5):728–34.
- Vu NT, Maher L, Zablotska I. Amphetamine-type stimulants and HIV infection among men who have sex with men: Implications on HIV research and prevention from a systematic review and meta-analysis. Journal of the International AIDS Society. 2015;18:19273.
- Guerra FM, Salway TJ, Beckett R, Friedman L, Buchan SA. Review of sexualized drug use associated with sexually transmitted and blood-borne infections in gay, bisexual and other men who have sex with men. Drug and Alcohol Dependence. 2020;216(108237).
- Hanum N, Cambiano V, Sewell J, Rodger AJ, Nwokolo N, Asboe D, et al. Trends in HIV incidence between 2013–2019 and association of baseline factors with subsequent incident HIV among gay, bisexual, and other men who have sex with men attending sexual health clinics in England: A prospective cohort study. PLoS Medicine. 2021;18(6):e1003677.
- Jennings JM, Wagner J, Tilchin C, Schumacher CM, Thornton N, Hamill MM, et al. Methamphetamine use, syphillis, and specific online sex partner meeting venues are associated with HIV status among urban Black gay and bisexual men who have sex with men. Sexually Transmitted Diseases. 2021;48(8S):S32–S9.
- Nerlander LM, Hoots BE, Bradley H, Broz D, Thorson A, Paz-Bailey G. HIV infection among MSM who inject methamphetamine in 8 US cities. Drug and Alcohol Dependence. 2018;190:216–23.
- Grov C, Westmoreland D, Morrison C, Carrico AW, Nash D. The crisis we are not talking about: One-in-three annual HIV seroconversions among sexual and gender minorities were persistent methamphetamine users. Journal of Acquired Immune Deficiency Syndromes. 2020;85(3):272.
- John SA, Parsons JT, Rendina HJ, Grov C. Club drug users had higher odds of reporting a bacterial STI compared with non-club drug users: Results from a cross-sectional analysis of gay and bisexual men on HIV pre-exposure prophylaxis. Sexually Transmitted Infections. 2019;95(8):626–8.
- Kohli M, Hickson F, Free C, Reid D, Weatherburn P. Cross-sectional analysis of chemsex drug use and gonorrhoea diagnosis among men who have sex with men in the UK. Sexual Health. 2019;16(5):464–72.
- Flores Anato JL, Panagiotoglou D, Greenwald ZR, Blanchette M, Trottier C, Vaziri M, et al. Chemsex and incidence of sexually transmitted infections among Canadian pre-exposure prophylaxis (PrEP) users in the l’Actuel PrEP Cohort (2013–2020). Sexually Transmitted Infections. 2022;98(8):549–56.
- Viamonte M, Ghanooni D, Reynolds JM, Grov C, Carrico AW. Running with scissors: A systematic review of substance use and the pre-exposure prophylaxis care continuum among sexual minority men. Current HIV/AIDS Reports. 2022;19(4):235–50.
- Gebru NM, Canidate SS, Liu Y, Schaefer SE, Pavila E, Cook RL, et al. Substance use and adherence to HIV pre-exposure prophylaxis in studies enrolling men who have sex with men and transgender women: A systematic review. AIDS and Behavior. 2023;27(7):2131–62.
- Moran A, Javanbakht M, Mimiaga M, Shoptaw S, Gorbach PM. Association of partnership-level methamphetamine use on inconsistent PrEP care engagement among GBMSM in Los Angeles County. AIDS & Behavior. 2023;04:04.
- Liu Y, Meng F-Z, Wang X, Wang P, Liu J-B, Hu W-H, et al. Methamphetamine facilitates HIV infection of primary human monocytes through inhibiting cellular viral restriction factors. Cell & Bioscience. 2021;11(1):1–9.
- Mata MM, Napier TC, Graves SM, Mahmood F, Raeisi S, Baum LL. Methamphetamine decreases CD4 T cell frequency and alters pro-inflammatory cytokine production in a model of drug abuse. European Journal of Pharmacology. 2015;752:26–33.
- Lawson KS, Prasad A, Groopman JE. Methamphetamine enhances HIV-1 replication in CD4+ T-cells via a novel IL-1β auto-regulatory loop. Frontiers in Immunology. 2020;11:136.
- Passaro RC, Pandhare J, Qian H-Z, Dash C. The complex interaction between methamphetamine abuse and HIV-1 pathogenesis. Journal of Neuroimmune Pharmacology. 2015;10:477–86.
- Knight R, Karamouzian M, Carson A, Edward J, Carrieri P, Shoveller J, et al. Interventions to address substance use and sexual risk among gay, bisexual and other men who have sex with men who use methamphetamine: A systematic review. Drug and Alcohol Dependence. 2019;194:410–29.
- Health Canada. Methamphetamine. 2023. Available from: https://www.canada.ca/en/health-canada/services/substance-use/controlled-illegal-drugs/methamphetamine.html Accessed October 30, 2023.
- Hoj SB, Minoyan N, Zang G, Larney S, Bruneau J. Gender, sexual orientation identity, and initiation of amphetamine injecting among people who inject drugs: Examination of an expanding drug era in Montreal, Canada, 2011–19. Drug & Alcohol Dependence. 2023;251:110956.
- Bach P, Hayashi K, Milloy MJ, Nosova E, Kerr T, Wood E, et al. Characterising the increasing prevalence of crystal methamphetamine use in Vancouver, Canada, from 2006–2017: A gender‐based analysis. Drug and Alcohol Review. 2020;39(7):932–40.
- Rivera AV, Harriman G, Carrillo SA, Braunstein SL. Trends in methamphetamine use among men who have sex with men in New York City, 2004–2017. AIDS and Behavior. 2021;25(4):1210–8.
- Palamar JJ, Han BH, Keyes KM. Trends in characteristics of individuals who use methamphetamine in the United States, 2015–2018. Drug and Alcohol Dependence. 2020;213:108089.
- Bourne A, Ong J, Pakianathan M. Sharing solutions for a reasoned and evidence-based response: Chemsex/party and play among gay and bisexual men. Sexual Health. 2018;15(2):99–101.
- Tomkins A, George R, Kliner M. Sexualised drug taking among men who have sex with men: A systematic review. Perspectives in Public Health. 2019;139(1):23–33.
- Stuart D. Chemsex: Origins of the word, a history of the phenomenon and a respect to the culture. Drugs and Alcohol Today. 2019;19(1):3–10.
- Strong C, Huang P, Li C-W, Ku SW-W, Wu H-J, Bourne A. HIV, chemsex, and the need for harm-reduction interventions to support gay, bisexual, and other men who have sex with men. Lancet HIV. 2022 Oct;9(10):e717–e725.
- Maxwell S, Shahmanesh M, Gafos M. Chemsex behaviours among men who have sex with men: A systematic review of the literature. International Journal of Drug Policy. 2019;63:74–89.
- Edmundson C, Heinsbroek E, Glass R, Hope V, Mohammed H, White M, et al. Sexualised drug use in the United Kingdom (UK): A review of the literature. International Journal of Drug Policy. 2018;55:131–48.
- Amundsen E, Muller AE, Reierth E, Skogen V, Berg RC. Chemsex among men who have sex with men: A systematic scoping review of research methods. Journal of Homosexuality. 2023: Mar 1–27.
- McElhiney M. Methamphetamine, MSM, and HIV. 2022. Available from: https://nahewd.org/wp-content/uploads/2022/09/Meth-MSM-HIV-McElhiney-7.01.2022.pdf Accessed November 2, 2023.
- Sousa AFL, Camargo ELS, Mendes IAC. Chemsex and its repercussions on the health of men who have sex with men (MSM): A global health perspective. Revista Brasileira de Enfermagem. 2023;76(3):e20230004.
- Harm Reduction International. Chemsex and harm reduction for gay men and other men who have sex with men. 2021 Available from: https://www.hri.global/files/2021/07/12/HRI_Briefing_Chemsex_July_2021_Final.pdf Accessed Novemer 10, 2023.
- Stanton AM, Wirtz MR, Perlson JE, Batchelder AW. “It’s how we get to know each other”: Substance use, connectedness, and sexual activity among men who have sex with men who are living with HIV. BMC Public Health. 2022;22(1):425.
- Treloar C, Hopwood M, Drysdale K, Lea T, Holt M, Dowsett GW, et al. Stigma as understood by key informants: A social ecological approach to gay and bisexual men’s use of crystal methamphetamine for sex. International Journal of Drug Policy. 2021;94:1–8.
- Gish A, Kiepek N, Beagan B. Methamphetamine use among gay men: An interpretive review of a non-sanctioned occupation. Journal of Occupational Science. 2020;27(1):26–38.
- Chawla N, Sarkar S. Defining “high-risk sexual behavior” in the context of substance use. Journal of Psychosexual Health. 2019;1(1):26–31.
- Nerlander LMC, Hoots BE, Bradley H, Broz D, Thorson A, Paz-Bailey G. HIV infection among MSM who inject methamphetamine in 8 US cities. Drug & Alcohol Dependence. 2018;190:216–23.
- Fletcher JB, Swendeman D, Reback CJ. Associations between major depressive episode, methamphetamine use disorder severity, and engagement in sexual risk-taking among methamphetamine-using men who have sex with men. AIDS and Behavior. 2018;22(5):1461–6.
- Bourne A, Reid D, Hickson F, Torres-Rueda S, Weatherburn P. Illicit drug use in sexual settings (‘chemsex’) and HIV/STI transmission risk behaviour among gay men in South London: Findings from a qualitative study. Sexually Transmitted Infections. 2015;91(8):564–8.
- Siddiq M, O’Flanagan H, Richardson D, Llewellyn CD. Factors associated with sexually transmitted shigella in men who have sex with men: A systematic review. Sexually Transmitted Infections. 2023;99(1):58–63.
- Hart TA, Noor SW, Tavangar F, Berlin GW, Skakoon-Sparling S, Tan DH, et al. Crystal methamphetamine use and bacterial sexually transmitted infections (STIs) among gay, bisexual and other sexual minority men in Canada. Drug and Alcohol Dependence. 2023;242:1–8.
- Baggaley RF, White RG, Boily M-C. HIV transmission risk through anal intercourse: Systematic review, meta-analysis and implications for HIV prevention. International Journal of Epidemiology. 2010;39(4):1048–63.
- Jin F, Prestage GP, Mao L, Poynten IM, Templeton DJ, Grulich AE, et al. “Any condomless anal intercourse” is no longer an accurate measure of HIV sexual risk behavior in gay and other men who have sex with men. Frontiers in Immunology. 2015;6:86.
- Murchu EO, Marshall L, Teljeur C, Harrington P, Hayes C, Moran P, et al. Oral pre-exposure prophylaxis (PrEP) to prevent HIV: A systematic review and meta-analysis of clinical effectiveness, safety, adherence and risk compensation in all populations. BMJ Open. 2022;12(5):e048478.
- Maxwell S, Shahmanesh M, Gafos M. Pre-exposure prophylaxis (PrEP) uptake and adherence experiences of gay and bisexual men who engage in chemsex: A qualitative study. International Journal of Drug Policy. 2022;103:1–6.
- Hammoud MA, Jin F, Maher L, Bourne A, Haire B, Saxton P, et al. Biomedical HIV protection among gay and bisexual men who use crystal methamphetamine. AIDS and Behavior. 2020;24(5):1400–13.
- Roux P, Fressard L, Suzan-Monti M, Chas J, Sagaon-Teyssier L, Capitant C, et al. Is on-demand HIV pre-exposure prophylaxis a suitable tool for men who have sex with men who practice chemsex? Results from a substudy of the ANRS-IPERGAY trial. Journal of Acquired Immune Deficiency Syndromes. 2018;79(2):e69–e75.
- O’Halloran C, Rice B, White E, Desai M, Dunn DT, McCormack S, et al. Chemsex is not a barrier to self-reported daily PrEP adherence among PROUD study participants. International Journal of Drug Policy. 2019;74:246–54.
- Hoenigl M, Jain S, Moore D, Collins D, Sun X, Anderson PL, et al. Substance use and adherence to HIV preexposure prophylaxis for men who have sex with men. Emerging Infectious Diseases. 2018;24(12):12.
- Okafor CN, Hucks-Ortiz C, Hightow-Weidman LB, Magnus M, Emel L, Beauchamp G, et al. Brief report: Associations between self-reported substance use behaviours and PrEP acceptance and adherence among Black MSM in the HPTN 073 study. Journal of Acquired Immune Deficiency Syndromes. 2020;85(1):23–9.
- Hojilla JC, Vlahov D, Glidden DV, Amico KR, Mehrotra M, Hance R, et al. Skating on thin ice: Stimulant use and sub-optimal adherence to HIV pre-exposure prophylaxis. Journal of the International AIDS Society. 2018;21(3):e25103.
- Jin H, Ogunbajo A, Mimiaga MJ, Duncan DT, Boyer E, Chai P, et al. Over the influence: The HIV care continuum among methamphetamine-using men who have sex with men. Drug and Alcohol Dependence. 2018;192:125–8.
- Passaro R, Ramsey K, Segura ER, Lake JE, Reback CJ, Clark JL, et al. Speed kills: Associations between methamphetamine use, HIV infection, tobacco use, and accelerated mortality among gay and bisexual men in Los Angeles, CA 20 years after methamphetamine dependence treatment. Drug and Alcohol Dependence. 2019;195:164–9.
- Carrico AW, Hunt PW, Neilands TB, Dilworth SE, Martin JN, Deeks SG, et al. Stimulant use and viral suppression in the era of universal antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes. 2019;80(1):89.
- Gorbach PM, Javanbakht M, Ragsdale A, Bolan RB, Flynn R, Mandler R, et al. Methamphetamine injection among young men who have sex with men: Risk for human immunodeficiency virus transmission in a Los Angeles cohort. Journal of Infectious Diseases. 2020;222(Suppl 5):S471–S6.
- Fulcher JA, Javanbakht M, Shover CL, Ragsdale A, Brookmeyer R, Shoptaw S, et al. Comparative impact of methamphetamine and other drug use on viral suppression among sexual minority men on antiretroviral therapy. Drug and Alcohol Dependence. 2021;221.
- Fulcher JA, Shoptaw S, Makgoeng SB, Elliott J, Ibarrondo FJ, Ragsdale A, et al. Brief report: Recent methamphetamine use is associated with increase rectal mucosal inflammatory cytokines, regardless of HIV-1 serostastus. Journal of Acquired Immune Deficiency Syndromes. 2018;78(1):119–23.
- Samikkannu T, Rao KV, Arias AY, Kalaichezian A, Sagar V, Yoo C, et al. HIV infection and drugs of abuse: Role of acute phase proteins. Journal of Neuroinflammation. 2013;10(1):1–7.
- Skowronska M, McDonald M, Velichkovska M, Leda AR, Park M, Toborek M. Methamphetamine increases HIV infectivity in neural progenitor cells. Journal of Biological Chemistry. 2018;293(1):296–311.
- Fattakhov N, Torices S, Stangis M, Park M, Toborek M. Synergistic impairment of the neurovascular unit by HIV-1 infection and methamphetamine use: Implications for HIV-1-associated neurocognitive disorders. Viruses. 2021;13(9):1883.
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(1):1–14.
- Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I, et al. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS and Behavior. 2006;10:185–90.
- Kesby JP, Heaton RK, Young JW, Umlauf A, Woods SP, Letendre SL, et al. Methamphetamine exposure combined with HIV-1 disease or gp120 expression: comparison of learning and executive functions in humans and mice. Neuropsychopharmacology. 2015;40(8):1899–909.
- Paolillo EW, Saloner R, Montoya JL, Campbell LM, Pasipanodya EC, Iudicello JE, et al. Frailty in comorbid HIV and lifetime methamphetamine use disorder: Associations with neurocognitive and everyday functioning. AIDS Research and Human Retroviruses. 2019;35(11–12):1044–53.
- Teodorof-Diedrich C, Spector SA. Human immunodeficiency virus type 1 and methamphetamine-mediated mitochondrial damage and neuronal degeneration in human neurons. Journal of Virology. 2020;94(20).
- Sun-Suslow N, Saloner R, Serrano V, Umlauf A, Morgan EE, Ellis RJ, et al. Lifetime methamphetamine use disorder and reported sleep quality in adults living with HIV. AIDS and Behavior. 2020;24:3071–82.
- Jones DL, Carrico AW, Babayigit S, Rodriguez VJ, Aguila C, Kumar M. Methamphetamine-associated dysregulation of the hypothalamic-pituitary-thyroid axis. Journal of Behavioral Medicine. 2018;41(6):792–7.
- McGowan I, Elliott J, Fuerst M, Taing P, Boscardin J, Poles M, et al. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. Journal of Acquired Immune Deficiency Syndromes. 2004;37(2):1228–36.
- Carrico AW, Cherenack EM, Roach ME, Riley ED, Oni O, Dilworth SE, et al. Substance-associated elevations in monocyte activation among methamphetamine users with treated HIV infection. AIDS. 2018;32(6):767–71.
- Fulcher JA, Hussain SK, Cook R, Li F, Tobin NH, Ragsdale A, et al. Effects of substance use and sex practices on the intestinal microbiome during HIV-1 infection. Journal of Infectious Diseases. 2018;218(10):1560–70.
- Cook RR, Fulcher JA, Tobin NH, Li F, Lee DJ, Woodward C, et al. Alterations to the gastrointestinal microbiome associated with methamphetamine use among young men who have sex with men. Scientific Reports. 2019;9(1):14840.
- Chahine A, Koru-Sengul T, Feaster DJ, Dilworth SE, Antoni MH, Klatt N, et al. Blue Monday: Co-occurring stimulant use and HIV persistence preduct dysregulated catecholamine synthesis. Journal of Acquired Immune Deficiency Syndromes. 2021;86(3):353–60.
- Ridpath A, Greene SK, Robinson BF, Weiss D. Risk factors for serogroup C meningococcal disease during outbreak among men who have sex with men, New York City, New York, USA. Emerging Infectious Diseases. 2015;21(8):1458–61
- Incera-Fernandez D, Gamez-Guadix M, Moreno-Guillen S. Mental health symptoms associated with sexualized drug use (chemsex) among men who have sex with men: A systematic review. International Journal of Environmental Research & Public Health. 2021;18(24):17.
- Colyer S, Light L. The Ontario HIV Treatment Network Cohort Study (OCS). Crystal methamphetamine use among GBMSM in the OCS, 2020–2021. [Presentation].
- Fletcher JB, Clark KA, Reback CJ. Depression and HIV transmission risk among methamphetamine-using men who have sex with men. Addiction Research & Theory. 2021;29(3):263–70.
- Moreno-Gamez L, Hernandez-Huerta D, Lahera G. Chemsex and psychosis: A systematic review. Behavioral Sciences. 2022;12(12):15.
- Strasser M, Halms T, Ruther T, Hasan A, Gertzen M. Lethal lust: Suicidal behavior and chemsex—A narrative review of the literature. Brain Sciences. 2023;13(2):20.
- Whitlock GG, Protopapas K, Bernardino JI, Imaz A, Curran A, Stingone C, et al. Chems4EU: Chemsex use and its impacts across four European countries in HIV-positive men who have sex with men attending HIV services. HIV Medicine. 2021;22(10):944–57.
- Hibbert MP, Hillis A, Brett CE, Porcellato LA, Hope VD. A narrative systematic review of sexualised drug use and sexual health outcomes among LGBT people. International Journal of Drug Policy. 2021;93:1–9.
- Goldsmith D, Hillyard M. The lack of focus on trans women in a themed issue of the International Journal of Drug Policy on sexualised drug use. International Journal of Drug Policy. 2019;68:1–2.
- Santos GM, Rapues J, Wilson EC, Macias O, Packer T, Colfax G, et al. Alcohol and substance use among transgender women in San Francisco: Prevalence and association with human immunodeficiency virus infection. Drug and Alcohol Review. 2014;33(3):287–95.
- Turner CM, Santos G-M, Arayasirikul S, Wilson EC. Psychosocial predictors of engagement in sexual risk behavior among trans* female youth ages 16–24 years in San Francisco. Journal of Acquired Immune Deficiency Syndromes. 2017;74(3):258.
- The Ontario HIV Treatment Network. Communicating the harms of methamphetamine use to men who have sex with men. 2020. Available from: https://www.ohtn.on.ca/wp-content/uploads/2020/12/RR154_meth-comm.pdf Accessed November 13, 2023.
- Nanin JE, Parsons JT, Bimbi DS, Grov C, Brown JT. Community reactions to campaigns addressing crystal methamphetamine use among gay and bisexual men in New York City. Journal of Drug Education. 2006;36(4):297–315.
- Siefried KJ, Acheson LS, Lintzeris N, Ezard N. Pharmacological treatment of methamphetamine/amphetamine dependence: A systematic review. CNS Drugs. 2020;34(4):337–65.
- Colfax GN, Santos G-M, Das M, Santos DM, Matheson T, Gasper J, et al. Mirtazapine to reduce methamphetamine use: A randomized controlled trial. Archives of General Psychiatry. 2011;68(11):1168–75.
- Coffin PO, Santos G-M, Hern J, Vittinghoff E, Walker JE, Matheson T, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: A placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246–55.
- Bakouni H, Sharafi H, Bahremand A, Drouin S, Ziegler D, Bach P, et al. Bupropion for treatment of amphetamine-type stimulant use disorder: A systematic review and meta-analysis of placebo-controlled randomized clinical trials. Drug and Alcohol Dependence. 2023; 1:253:111018.
- Brown HD, DeFulio A. Contingency management for the treatment of methamphetamine use disorder: A systematic review. Drug and Alcohol Dependence. 2020;216:108307.
- Carrico AW, Gomez W, Jain J, Shoptaw S, Discepola MV, Olem D, et al. Randomized controlled trial of a positive affect intervention for methamphetamine users. Drug and Alcohol Dependence. 2018;192:8–15.
- Corsi KF, Shoptaw S, Alishahi M, Booth RE. Interventions to reduce drug use among methamphetamine users at risk for HIV. Current HIV/AIDS Reports. 2019;16:29–36.
- Zhang S, Shoptaw S, Reback CJ, Yadav K, Nyamathi AM. Cost-effective way to reduce stimulant-abuse among gay/bisexual men and transgender women: A randomized clinical trial with a cost comparison. Public Health. 2018;154:151–60.
- Gómez W, Olem D, Andrews R, Discepola MV, Ambrose P, Dilworth SE, et al. Optimizing contingency management with methamphetamine-using men who have sex with men. Cognitive and Behavioral Practice. 2018;25(2):286–95.
- Parsons JT, Lelutiu-Weinberger C, Botsko M, Golub SA. A randomized controlled trial utilizing motivational interviewing to reduce HIV risk and drug use in young gay and bisexual men. Journal of Consulting and Clinical Psychology. 2014;82(1):9.
- Parsons JT, John SA, Millar BM, Starks TJ. Testing the efficacy of combined motivational interviewing and cognitive behavioral skills training to reduce methamphetamine use and improve HIV medication adherence among HIV-positive gay and bisexual men. AIDS and Behavior. 2018;22(8):2674–86.
- Mimiaga MJ, Reisner SL, Pantalone DW, O’cleirigh C, Mayer KH, Safren SA. A pilot trial of integrated behavioral activation and sexual risk reduction counseling for HIV-uninfected men who have sex with men abusing crystal methamphetamine. AIDS Patient Care and STDs. 2012;26(11):681–93.
- Mimiaga MJ, Pantalone DW, Biello KB, Hughto JM, Frank J, O’Cleirigh C, et al. An initial randomized controlled trial of behavioral activation for treatment of concurrent crystal methamphetamine dependence and sexual risk for HIV acquisition among men who have sex with men. AIDS Care. 2019;31(9):1083–95.
- Reback CJ, Grant DL, Fletcher JB, Branson CM, Shoptaw S, Bowers JR, et al. Text messaging reduces HIV risk behaviors among methamphetamine-using men who have sex with men. AIDS and Behavior. 2012;16:1993–2002.
- Ameri A, Keshvardoost S, Bahaadinbeigy K. Impact of mobile phone-based interventions on methamphetamine use and high-risk sexual behaviours in men who have sex with men (MSM): A systematic review. Addiction & Health. 2020;12(1):58–68.
- Carrico AW, Flentje A, Gruber VA, Woods WJ, Discepola MV, Dilworth SE, et al. Community-based harm reduction substance abuse treatment with methamphetamine-using men who have sex with men. Journal of Urban Health. 2014;91:555–67.
Suggested citation
Rapid Response Service. Health outcomes of and interventions to reduce methamphetamine use among men who have sex with men. Toronto, ON: The Ontario HIV Treatment Network; March 2024.
Prepared by
Danielle Giliauskas and David Gogolishvili
Illustration credit
Rachel Chung