Questions
What is the impact of nonadherence to antiretroviral therapy (ART) on population-level health outcomes?
Key take-home messages
- Several studies analyze overall healthcare costs, pharmacy costs, and cost savings among people living with HIV relative to level of ART adherence (1–5).
- Generally, adherence to ART appears to predict levels of healthcare utilization, with studies reporting that poorer adherence to ART results in increased utilization (6, 7), and better adherence results in decreased utilization (3, 4, 8).
- Suboptimal adherence to ART appears to contribute to a higher risk of mortality (9–11).
- Poor adherence to ART in pediatric HIV care may continue into adulthood and in some cases, can contribute to mortality (12, 13).
- Acquired HIV drug resistance can occur when an individual living with HIV has poor adherence to ART (14), though this can vary, depending on the class of antiretrovirals in the prescribed regimen (15).
The issue and why it’s important
While HIV is a chronic condition that can be managed through antiretroviral therapy (ART) (16), it is critical that treatment be adhered to (1, 17). Though earlier treatment regimens were complex (18) and included “high pill burdens and/or complicated administration protocols” (19), treatment options have significantly expanded (20). Modern treatments are “highly efficacious, well-tolerated, [and] safe” (21). Additionally, newer therapy includes fixed-dose combination treatment (also known as a single-tablet regimen: one pill, taken once daily, with at least two active agents) (20, 22) which has shown to improve adherence when compared to multiple-tablet regimens (23–25).
The goals of ART are to prevent onward transmission of the virus, restore or retain immune function, and suppress viral replication (19). By curbing viral replication, HIV disease progression is prevented, as are HIV-related morbidity and mortality (19). However, ART is not curative: it must be taken indefinitely to sustain suppression of HIV (19). Oftentimes, near-perfect adherence of ≥95% to medication is cited as necessary for viral suppression, though some research has found that this may be lower, and can vary depending on the regimen (26). Nevertheless, long-term adherence to ART remains a challenge for a variety of reasons (27, 28), including adverse drug reactions (29), depressive symptoms/disorder (30), lack of adequate housing (31), and food insecurity (32). Indeed, suboptimal adherence is complex (33) and interventions to improve adherence to ART have been extensively examined in the literature (34–42).
While adherence to treatment has demonstrated positive outcomes among people living with HIV (43–45), nonadherence occurs universally, in both high- and low-income settings (27). One meta-analysis of 84 observational studies across twenty countries worldwide found that on average, 62% of individuals reported ≥90% adherence to ART (46). Individuals who were able to maintain ≥90% adherence were more likely to be from studies with: higher proportions of men who have sex with men, lower proportions of injection drug users, individuals at an earlier stage of infection, and in countries with lower Human Development Index values (46). In Canada, one estimate suggests that adherence to ART among people living with HIV is on average around 93% (based on data from 2010–2019) (47); another estimate from 2015 suggests that 94% of Ontarians on ART are virally suppressed (48). However, it should be noted that there are disparities in rates of viral suppression among different population groups in Canada (49). The effects of nonadherence to ART on individual-level outcomes, such as viral load (50) and CD4 count (51), are well-documented in the literature. This review focuses on the effects of nonadherence to ART on population outcomes, which encompasses the overall health of a population and how it is distributed among various groups (52).
What we found
While adherence to HIV medications should be a part of routine clinical care for people living with HIV (53), the definition of adherence is broad, as are the means of its monitoring (27). Generally, adherence can be described as “[t]aking HIV medicines every day and exactly as prescribed” (54). Currently, there is no “gold standard” to measure adherence to ART (53, 55). However, there are five main ways adherence to ART can be measured: by self-report, medication event monitoring systems, pill count, pharmacy refill, and therapeutic drug monitoring (53). All of these methods are utilized in the studies identified in this review and are described in more detail henceforth.
Additionally, the literature reporting outcomes to ART nonadherence included in this review used data from a broad range of years: 1996 to 2019. During this time, aspects of ART have changed considerably. One study from 2014 by Tseng et al. illustrates the timeline of antiretroviral drug development, noting that from 1987 to 2013, more than 25 drugs were developed for the treatment of HIV (some of which are no longer in use) (56). However, as of February 2021, more than 40 drugs are used for the treatment of HIV in the U.S. (57). Tseng et al. state that “[t]reatment of HIV has evolved from gruelling regimens with high pill burden, inconvenient dosing, treatment-limiting toxicities, food and drug interactions, incomplete viral suppression and emergence of drug resistance to manageable one or two pill once daily regimens that can be initiated in early HIV disease and continued with control of viral replication over much of an individual’s lifespan” (56).
Because the studies in this review include various measurements of adherence over a broad date range, findings should be interpreted with this caveat in mind. Nevertheless, nonadherence to ART appears to impact healthcare utilization and costs, mortality, and viral resistance to ART. A few other studies examine the relationship between nonadherence and sexual risk behaviours, though this literature is not as robust. The following sections detail the studies that examined the aforementioned population-level outcomes.
Impact of ART nonadherence on healthcare utilization and costs
A 2019 retrospective study from the U.S. found that participants with varying levels of ART adherence also had different levels of healthcare utilization, demographics, and overall costs (1). Medical and pharmacy claims information from a database for Medicaid patients (n=332) and commercially insured patients (n=1,698) enrolled for ≥6 months before and ≥15 months after the index date were analyzed (1). The index date refers to the date of the first medical claim with an HIV diagnosis, or claim for HIV medication, occurring between the study dates of July 2007 and June 2014 (1). Participants were categorized by pill burden (i.e. single-table regimen or multiple-tablet regimen) and adherence by proportion of days covered: ≥95%, highly adherent or <95%, less adherent (1). Commercially insured patients were further divided into two cohorts based on availability of follow-up data: <3 years available (n=686) or ≥3 years available (n=1,012) (1). For all commercially insured patients, those with single-tablet regimens were more adherent compared to those on multiple-tablet regimens (1). Among all commercially insured patients, those who were less adherent had lower pharmacy costs and higher mean annual medical costs (excluding pharmacy costs) compared to highly adherent patients (1). Furthermore, among highly adherent patients, cost savings were realized in mean annual inpatient costs: USD 2,525 cost savings in the <3-year follow-up cohort (p= 0.0003) vs. nonadherent patients and USD 815 cost savings in the ≥3-year follow-up cohort (p< 0.001) vs. nonadherent patients. Overall, the total costs (i.e. medical costs and pharmacy costs) were higher for individuals who were highly adherent compared to those who were less adherent, though this finding was not statistically significant and may be attributed to higher pharmacy costs for highly adherent patients (1). Authors also note that participants with Medicaid insurance had lower rates of ART adherence and more comorbidities compared to those who were commercially insured (1).
A retrospective observational cohort study (2016) using data from 2006–2013 in commercial and Medicaid databases compared the persistence (i.e. time to treatment discontinuation or modification), adherence, and all-cause total healthcare costs among people living with HIV initiating ART (specifically, atazanavir boosted with ritonavir and darunavir boosted with ritonavir) (58). Individuals were followed from the time they started the regimen, until discontinuation (≥30 day gap in ART) or initiation of new ART medication (58). During this time, per-patient per-month healthcare costs (including insurer- and patient-paid portions) and adherence were measured (58). Adherence was measured in two ways – proportionally, from 0–100%, and as a categorical variable, based on proportion of days covered (e.g. ≥80%) (58). The final unmatched study sample included 4,303 commercial and 2,967 Medicaid participants (58). In order to achieve balance between both cohorts, propensity score matching was used (58), a statistical technique for reducing or eliminating the effects of measured confounders in observational studies (59). After propensity score matching, the sampled yielded 2,988 commercially-insured and 1,518 Medicaid-insured participants (58). In the follow-up period, 85–95% of participants in both cohorts had a proportion of days covered of ≥80%; 50–66% had a proportion of days covered of ≥90% (58). Authors note that the only marginally significant result in terms of adherence was that the odds of having a proportion of days covered ≥80% favoured atazanavir boosted with ritonavir among the ART-naïve Medicaid patients (58). Participants on atazanavir boosted with ritonavir had lower all-cause costs, but this was not statistically significant (58).
Another retrospective study (2015) examined Medicaid claims in Florida among people living with HIV and those diagnosed with AIDS, and the association of antiretroviral adherence to average monthly healthcare expenditures across a 24-month measurement (2). Claims data from July 2006 to June 2011 were utilized; all participants (n=502) were naïve to HIV treatment for at least 12 months prior to the measurement period (2). Participants were categorized based on medication possession ratios (≥90%, adherent; <90%, nonadherent) and according to status (HIV-positive or AIDS-diagnosed) (2). Overall, 78.7% of participants were nonadherent: among those HIV-positive, 75.9% (n=176) were nonadherent, and among those diagnosed with AIDS, 81.1% (n=219) were nonadherent (2). The average unadjusted monthly total healthcare expenditures were USD 1,847 for the nonadherent group (n=395) and USD 1,976 for the adherent group (n=107) (2). For those who were HIV-positive, the average unadjusted monthly total healthcare expenditures for the nonadherent group (n=176) were USD 1,280; for the adherent group (n=56), USD 1,938 (2). Authors had three general findings from the study. First, there was a statistically significant association between adherence and higher average healthcare costs among HIV positive participants; second, among AIDS-diagnosed individuals, adherence was not significantly associated with total health expenditures, in the short-term (2). Finally, no statistical association was found between medication adherence and total expenditures among adherent HIV-positive participants and all AIDS-diagnosed patients, suggesting no economic advantage (at least short-term) of preventing disease progression from HIV to AIDS; however, the authors note this finding is contrary to previously published literature and that there is a complex relationship between ART adherence and healthcare costs (2).
A third retrospective cohort study (2017) across 29 U.S. states examined if different levels of adherence to ART were associated with different clinical outcomes, such as emergency department visits and duration of hospital admission (6). The study was conducted among older people living with HIV (between 50–64 years of age), who received a diagnosis of symptomatic HIV or AIDS, were enrolled in Medicaid between January 2008 and November 2009, and who received ART during the study dates (6). Adherence to ART was measured by proportion of prescribed days covered, and was categorized by four levels: ≥95%, 90–94%, 80–89%, and <80%, where ≥95% was considered optimal (6). From the total sample size (n=5,177), 32.3% reported ≥95% adherence to ART (6). Participants who reported <80% adherence were significantly more likely to have emergency department visits, compared to those reporting ≥95%, though there was no statistical difference in emergency department visits for those reporting 80–89% and 90–94% adherence (6). Additionally, those reporting <80% adherence were also more likely to have a longer duration of hospital admission compared to those reporting ≥95% adherence (6). Participants who reported any comorbidities were more likely to report longer duration of hospital admission compared to those not reporting a comorbidity (6). Overall, participants who were <80% adherent to ART were 34% more likely to visit the emergency department and 25% more likely to report a prolonged hospital admission (6).
Another study (2012) from the U.S. examined the differences between pill burden and adherence, and the impact of adherence on hospitalization (8). Data for the analysis were from a national insurance claims database; participants were selected for inclusion if they received at least one HIV or AIDS diagnosis between June 2006 and December 2008, and had evidence of receipt of a complete ART regimen (8). Patients were followed from the start of their regimen until the earliest date of regimen discontinuation (defined as 90 consecutive days with no observed refills), disenrollment from the health plan, or the end of the database (which was March 2009) (8). The final study sample included 7,073 individuals, where 33% (n=2,365) were on a single-tablet regimen, 6% (n=411) were on a multi-tablet regimen of two pills per day, and 61% (n=4,297) were on a multi-tablet regimen of three or more pills per day (8). Participants who achieved ≥95% adherence to ART are as follows: single-tablet regimen, 47% (n=1,114); two or more pills per day, 41% (n=168); three or more pills per day, 34% (n=1,468) (8). Logistic regression models demonstrated that those on the single-tablet regimen were associated with a 59% greater likelihood of achieving ≥95% adherence to ART, when compared to those who were on a regimen of three or more pills per day (8). Additionally, there was a 24% lower risk of hospitalization among those on a single-tablet regimen compared to those taking three or more tablets per day (8). Finally, participants who achieved ≥95% adherence to ART had a lower rate of hospitalization compared to those who did not, regardless of pill burden (8).
A retrospective cohort study (2015) analyzing Medicaid claims from 14 states in the southern U.S. examined the impact of ART treatment adherence among people living with HIV and hepatitis C (n=4,115) between 2005–2007 (3). To determine adherence to ART, the proportion of prescribed days covered by the regimen was examined, defined as the total daily supply of ART divided by a follow-up period of 365 days (3). Adherence was stratified by person-years: <25%, 25–50%, 50–75%, 75–95%, and >95% (3). Authors found that as adherence decreased, hospitalization and visits to the emergency department increased (3). For mortality, those with <25% adherence had greater than four times the risk of death compared to those who had >95% adherence (OR 4.22; 95% CI 3.03, 5.87) (3). Relative to the group that had <25% adherence, cost per QALY (quality-adjusted life year) saved ranged from USD 21,874 (when adherence was increased to 25–50%) to USD 37,229 (when adherence increased to 75–95%) (3).
A conference presentation from 2012 developed a microsimulation model to examine the impact of ART nonadherence to 2060 in New Zealand (60). Microsimulation is a modelling technique that performs analysis at the level of the individual, capturing behaviour (60, 61). Thus, these models are used to simulate large representative populations of behaviours occurring at the level of the individual (61). Because the decision to adhere to medication occurs at the individual level, it can be used in microsimulation models (60). Adherence was operationalized as a dichotomous variable: adherent or nonadherent (60). Authors considered how nonadherence impacted treatment costs, hospitalization costs, deaths, and QALYs (60). Long-term, authors found that nonadherence increased hospitalization costs by NZD 8.2 million, and decreased treatment costs by NZD 169.0 million (60). Nonadherence resulted in an average of 22 more AIDS-related deaths per year, and reduced quality of life by 6,351 QALYs (60).
Another U.S. study (2011) among randomly selected participants (n=1,896) from the Veteran’s Health Administration (VHA) characterized the cost of HIV care, including cost of medication, from 2002–2006 (62). Of note, the U.S. VHA has lower pharmaceutical costs and more comprehensive benefits compared to other U.S. health plans (62). Pharmacy data was used to identify those who received ART (62). From the sample, 75.5% (n=1,431) received ART; of this, 33.5% (n=479) had enough medication dispensed to receive three drugs for 85% of days in the study year (62). The mean annual VHA care cost was USD 17,484; medication accounted for 33.9% of this (62). For highly adherent patients, 62.8% of total costs was for outpatient pharmacy; for patients with a lower level of adherence, 32.2% was for outpatient pharmacy; for patients who did not receive ART, 6.2% of total costs was for outpatient pharmacy (62). In general, those with higher medication costs had lower acute hospital costs (62).
A 2006 study utilized data from a hypothetical cohort of people living with HIV, on an initial treatment regimen, to quantify the long-term clinical and economic effects of nonadherence in routine practice and to estimate the cost-effectiveness of improving adherence to the levels in clinical trials (5). Authors developed a Markov model, which is used to represent disease processes that change over time, thus demonstrating disease progression (63). Authors found that “typical” adherence (i.e. based on data from observational studies) was associated with decreased life expectancy and decreased lifetime costs compared to “ideal” adherence (i.e. based on utilization observed in clinical trials) (5). If adherence was improved from “typical” to “ideal” levels, 1.2 QALYs on average would be gained at an incremental cost of USD 29,400 per QALY gained (not including the costs of an adherence-enhancing intervention) (5). When hypothetical intervention costs were considered, improving adherence from “typical” to “ideal” levels was found to be cost-effective, until the intervention exceeded USD 1,600 per patient per year (5). After USD 1,600, the cost-effectiveness ratio exceeded USD 50,000 per QALY gained, a threshold “commonly accepted at the minimum standard for cost-effective medical interventions in the U.S.” (5).
A retrospective cohort study from 2008 in Colorado examined the relationship between adherence to ART, health care utilization, and medical costs at an inner-city safety net hospital where HIV primary care is provided at an infectious disease clinic (4). All participants (n=325) were people living with HIV, naïve to ART, who initiated ART between the years of 1997 to 2003 (4). Adherence was measured using pharmacy refill data, and the billing database of the hospital was used to acquire healthcare utilization data and medical costs (4). Authors used quartiles of antiretroviral adherence to identify four groups of participants: the first quartile had 100% adherence, the second quartile had a median adherence of >97%; the third, 86.3%, and the fourth, 47.3% (4). In general, those who had better adherence to ART had lower healthcare utilization and higher medical costs (4). For healthcare utilization, the following interquartile comparisons were statistically significant: emergency room visits and hospital admission, per year, were higher for patients in the fourth (lowest) quartile compared to the first three quartiles, and patients in the fourth quartile also had a higher number of hospital days, per year, compared to those in quartiles one and two (4). For medical costs, there was a statistically significant association between annual costs and the adherence quartile: for those in the fourth quartile, medical costs were lower compared to the other three quartiles, due to a lower annual cost for ART (4). An older Canadian study from 2008 examined the impact of hospitalization and nonadherence among a group of individuals who initiated ART between the years of 1996–2001 (7). Participants were from British Columbia, and part of an observational cohort study for ART naïve adults living with HIV, who start on a regimen of three ART drugs (7). Adherence was estimated based on prescription refill or prescription compliance; patients were dichotomized as either “adherent” (>95% adherence to ART) or “nonadherent” (<95% adherent to ART) (7). Hospitalization events were defined as admission to any hospital in British Columbia for one or more days (7). Among the 1,605 participants in the cohort who were eligible for the study, 42% (n=672) had at least one hospitalization after they initiated ART; a greater proportion of nonadherent patients were hospitalized during the study period (64). Compared to the nonadherent participants, those who were classified as adherent were significantly less likely to have been hospitalized in the six months prior to baseline (7). Once confounders were controlled for, the overall adjusted model suggested that nonadherent participants were 1.88 times more likely to be hospitalized than adherent participants (95% CI: 1.60, 2.21) (7).
Being “not on ART” and healthcare utilization
We also identified two other studies that describe healthcare outcomes for individuals described as those “not on ART” or “not receiving ART”.
A study published in 2020 examined medical records of people living with HIV who received care at St. James’ Hospital in Dublin, Ireland, between 2014–2015 (65). The hospital functions as a university teaching hospital and as a tertiary HIV care referral centre (65). The study sought to characterize usage of unscheduled health care and attendance costs were described among “super-utilizers”, identified in this study as “…any patient who accrued >30 days of inpatient care during the 1-year study period” (65). The authors note that super-utilizers living with HIV typically have unscheduled hospital visits that are potentially preventable (65). Of the 2,043 people living with HIV who attended outpatient HIV care, 208 had ≥1 unscheduled inpatient admission during the study period; 22 had a cumulative stay of >30 days during the study period and were thus identified as super-utilizers (65). The 22 super-users accounted for 99 emergency department visits, 77 inpatient admissions, 1,581 inpatient bed-days, and 74 days in the intensive therapy unit; 18 of these 22 super-users had potentially preventable presentations (65). The most common reasons for inpatient admissions among super-utilizers was bacterial infections (65). Of the 22 super-utilizers, 20 were diagnosed with HIV at least nine years before the study period; among them, 13 were not engaged in outpatient care and were not on ART during the study period (65). Eighteen months after completion of the study period, ten of the 22 super-utilizers died: the relative risk of dying (within 30 months of commencing the study) of a super-utilizer was about 33.17 (95% confidence interval, 18.44–59.67; p<0.0001) when compared to the general cohort (65). The inpatient cost to the hospital of 20 super-utilizers (after excluding the two patients whose hospital admission was not HIV-related) was EUR 973,460 for one year (65).
One retrospective cohort study (2015) examining hospitalization rates among people living with HIV in Dallas, Texas, found that not receiving ART greatly increased the odds of a preventable readmission to hospital (66). Electronic medical records were used to identify all hospital readmissions that occurred within 30 days of discharge among hospitalized individuals living with HIV at an urban safety net hospital in 2011 (66). From the 1,137 index HIV admissions, 19% (n=213) resulted in 30-day readmissions (66). The total number of index HIV admissions was among 930 unique individuals, 15% (n=137) of who accounted for readmission (66). Seven of the 137 were excluded, as they did not constitute a “true” readmission, leaving 130 in the final sample (66). Of these remaining 130, 79 had one readmission, 27 had two, eight had three, and 16 had four or more readmissions (66). Patients with multiple readmissions were categorized as follows: advanced AIDS and recurrent AIDS-defining illness, patients with chronic conditions, and patients receiving chemotherapy for cancer (66). Two independent reviews examined whether readmissions were preventable, using published criteria and detailed chart review (66). From the 130 participants, 48% (n=62) of initial readmissions were deemed preventable (66).
Nonadherence and mortality
A 2013 study from New York City examined outcomes of hospitalized people living with HIV to describe and evaluate causes of death (11). Authors performed a retrospective chart review of all people living with HIV who died between 2004-2008 at St. Luke’s-Roosevelt Hospital Centre, a tertiary acute care hospital with a comprehensive HIV care program (11). Of the 9,101 people living with HIV who were hospitalized, 237 deaths were reported with a mortality rate of 2.6% (11). Of the 237 individuals who died, medical charts were available for 208 individuals (11). Median length of hospital stay was 16 days (11). Non-AIDS related illness, such as sepsis, non-recurrent bacterial pneumonia, and cardiac disease, among others, accounted for 81.7% of deaths (n=170); AIDS related illness, such as Pneumocystis jirovecii pneumonia, AIDS-related encephalopathy, Mycobacterium avium-intracellulare or Mycobacterium tuberculosis infections, among others, accounted for 18.3% of deaths (n=38) (11). Authors noted that there was a tendency of lower adherence rates to ART among participants in the AIDS mortality group (11).
A 2021 study among people living with HIV enrolled in the Swiss HIV Cohort Study (SHSC) examined the relationship between self-reported adherence to ART and cardiovascular events (including death) and non-cardiovascular-related death (9). Participants included in the study had initiated ART between 2003-2018, had viral suppression for ≥6 months, and no history of cardiovascular disease (n=6,971) (9). Adherence in the SHSC is measured by self-report using two questions: “How often did you miss a dose in the last four weeks?” and “Did you have a period of no drug intake for >24 hours in the last four weeks?” (9). Two definitions for incomplete adherence to ART were utilized: ≥1 and ≥2 missed doses in the past four weeks (9). A high adherence threshold of any missed doses (i.e. <100%) was used (9). In multivariate analyses, authors found that missing ≥1 dose was associated with a hazard ratio for non-cardiovascular-related mortality of 1.44 (95% CI, 1.00–2.07; p=0.05); missing ≥2 doses was associated with a hazard ratio for non-cardiovascular-related mortality of 2.21 (95% CI, 1.37–3.57; p=0.001) (9). Authors concluded that incomplete adherence to ART was significantly associated with increased risk for non-cardiovascular-related mortality among virally suppressed people living with HIV (9).
A 2015 study conducted among participants in the SHSC from 2003–2012 had similar findings (10). Using a prospective observational cohort study design, authors determined if nonadherence to ART impacted virologic failure and all-cause mortality in individuals starting ART for the first time (10). Participants were asked how often they missed a dose in the past four weeks: none, once a month, once every two weeks, once a week, more than once a week, and every day (10). Answers were converted to missing none, one, two, or more than two doses of ART in the past four weeks (10). The final data set was comprised of 3,155 individuals; 3.3% died (n=104), 36.6% (n=1,155) reported missing one dose, 13.1% (n=411) reported missing two doses, and 10.6% (n=333) reported missing more than two doses of ART (10). Missing more than two doses was associated with an increased risk of death (hazard ratio 4.87; 95% CI 2.21–10.73) (10).
Two studies examined outcomes of nonadherence to ART among adolescents and young adults who acquired HIV perinatally or parenterally (12, 13). This is a population that has unique challenges related to ART, including poorly controlled HIV due to suboptimal early regimens and nonadherence (67). A retrospective cohort study from Spain (2020) characterized mortality and comorbidities of adolescents after they transitioned to adult care (12). Adherence was estimated using two variables: the proportion of time that a patient had an undetectable viral load and the number of voluntary treatment interruptions, defined as missing two or more consecutive medical visits in six months (12). Of the 651 individuals who were transferred to adult care between 1995–2017, collected data was available for 401 participants (12). Of these 401 participants, 14 deaths (3.5%) were registered after transition to adult care; the transitions occurred between 1998 and 2010, and the deaths occurred between 2009 and 2019 (12). Of the individuals who died, adherence was deemed to be suboptimal since early childhood; additionally, the number of voluntary treatment interruptions was high when in pediatric care, and increased in adult care (12). Authors note that some participants, as they became older, improved their ART adherence, but died of advanced disease before the benefits of adherence to ART could be realized (12). A 2014 study from England examined deaths after young people living with HIV were transitioned to adult care (13). This multicentre audit sampled fourteen adult clinics caring for people living with HIV in 2011 (13). Eleven deaths were reported, with years ranging between 2003–2011; these deaths were matched to a clinical database of children living with HIV in the UK (13). Of the eleven deaths, nine had poor adherence in pediatric care which continued following transition, and were associated with advanced HIV disease (13). Authors note that those with poor adherence were offered multimodal support from various specialists, including clinical nurse specialists, community nurses, and health advisors (13).
Nonadherence and acquired HIV drug resistance
Acquired HIV drug resistance can occur when an individual living with HIV has: poor adherence to ART, treatment interruptions, inadequate drug concentrations, and/or suboptimal drug combinations (14). Acquired drug resistance is problematic as it can lead to poor health outcomes in the individual living with HIV, and can also be passed to others (resulting in transmitted HIV drug resistance) (14). While the relationship between poor adherence to ART and development of drug resistance is well-documented in the literature (15, 68–70), it should be noted that this relationship is complex (14).
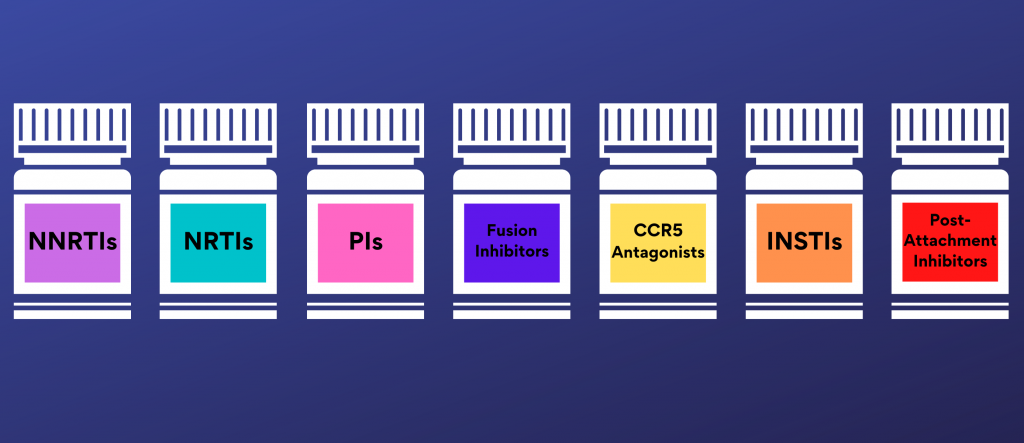
Furthermore, the relationship between ART adherence and drug resistance can vary, depending on the class of antiretrovirals in the prescribed regimen (15). Currently, there are seven types of antiretroviral HIV drugs, classified by how the drug interferes with the life cycle of HIV (71). These seven classes are: “nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), fusion inhibitors, CCR5 antagonists, post-attachment inhibitors, and integrase strand transfer inhibitors (INSTIs)” (71). Some of these classes are referenced in the following studies detailing resistance.
One study from 2020 reviewed published cases of resistance to the HIV drug dolutegravir (INSTI) as virologic failure among those treatment-naïve and treatment-experienced (but naïve to INSTIs) has been reported in clinical settings (72). Dolutegravir-based ART is a preferred first- and second-line treatment regimen for people living with HIV due to its efficacy, safety, and high barrier to drug resistance (72). Of the fifteen cases of treatment failure identified, ten had suboptimal levels of dolutegravir when measured, or had documented poor adherence (72). Authors concluded that poor adherence and HIV disease factors such as high baseline viral load and opportunistic infections may contribute to emergent drug resistance (72).
A retrospective study from 2014 examined if resistance to regimens containing the PI atazanavir (ATV) boosted with ritonavir (ATV/r) was associated with unscheduled treatment interruptions (defined as discontinuation of all ART drugs for any period of time) (73). Data was extracted from medical records of participants (n=202) between 2004–2009 (73). In more than half of participants, the most common reason for unscheduled treatment interruptions was nonadherence (73). The study did not observe major PI resistance mutations to ATV/r, despite some patients reporting up to six unplanned treatment interruptions; however, some minor mutations were observed (73).
One longitudinal retrospective cohort study from British Columbia published in 2018 sought to identify long-term trends in drug resistance before and after initiation of ART among people living with HIV (74). The individuals included in this study (n=6,543) initiated ART between 1996–2014, which consisted of two NTRIs and an NNRTI, a PI or an alternative; information on drug resistance was collected until 2016 (74). Participants’ CD4 count and plasma viral load were measured six months before ART was initiated (74). The primary outcome was detection of drug resistance mutations in participants’ plasma viral load samples (74). From the initial sample, a subset (n=682) had transmitted drug resistance at baseline (74). Of those who did not have baseline resistance (n=5,861), 19% (n=1,092) had the primary outcome of detectable resistance (74). Authors found that the highest odds of developing acquired drug resistance between 1996–2010 were individuals who were 60%–80% adherent to their ART regimen (74). This shifted in 2011–2014, where individuals with the highest odds of developing acquired drug resistance were those with <40% adherence to ART; authors note that this lower figure may be due to increasing antiretroviral efficacy in more recent years (74). Furthermore, authors identified a substantial decline in acquired drug resistance throughout the study period of nearly 20 years, suggesting that in recent years, acquired resistance is mainly observed among those with the lowest levels of adherence to ART (74).
An international trial of individuals on second-line ART found that poor adherence was a determinant of virological failure (75). The randomized controlled trial from 2015 randomly enrolled participants with virological failure after being on first-line ART, which consisted of an NNRTI, plus two nucleotide reverse transcriptase inhibitors (75) (NtRTIs, part of the NRTI drug class (10, 54). Data was collected between 2010–2013; eligible participants were randomly assigned to two groups of second-line ART: the “NtRTI group” received ritonavir boosted with lopinavir given with either two or three NtRTIs, and the “raltegravir group” received ritonavir boosted with lopinavir boosted with raltegravir (75). Adherence was measured at week four and 48 with a questionnaire, asking participants if they took all pills or less than all pills in the last seven days (75). At 96 weeks, 240 participants in the NtRTI group had viral load measurements and baseline sequence data was available for 215; of these, 75 had virological failure and acquired resistance mutations occurred in 11 participants (75). At 96 weeks, 255 participants in the raltegravir group had viral load measurements and baseline sequence data was available for 236; of these, 80 had virologic failure and acquired resistance occurred in 23 participants (75). Authors found that “less than complete” adherence at week four and week 48 was a determinant of virologic failure (75).
A study from 2013 examined a cohort of patients starting a new class of ART between 2003–2010 in Switzerland (76). A clinical database was screened to determine if genotypic drug resistance tests (GRT) were performed while patients received ART, and if this could be linked to an adherence assessment (76). Participants included in the study had to be either: ART naïve and initiating a PI boosted with ritonavir (PI/r) regimen, initiating an NNRTI regimen, or failed a first-line treatment regimen and initiated a second regimen with two active drugs that contained a drug class different than the initial regimen (76). Adherence was measured twice yearly via self-report on the frequency of missed doses over the past four weeks, and categorized as 100%, 95–99%, and <95% adherence (76). GRT results from 314 individuals on ART were examined: 162 individuals were receiving NNRTI, and 152 were receiving PI/r (76). Adherence between the two groups was similar, with 85% reporting <95% adherence (76). Generally, the number of new mutations increased as nonadherence increased (76). For those on the NNRTI regimen, the emergence of any mutation (as defined by the International Antiviral Society in the U.S.) was significantly associated with nonadherence (76). For those on the PI regimen, nonadherence was significantly associated with development of a single new mutation (76). A study from 2011 conducted in Italy examined the correlations between adherence to ART and drug-related mutations among people living with HIV displaying treatment failure (n=40) (77). Adherence was measured by self-report, and authors calculated the percentage of adherence for doses missed in the past week, past month, and past three months (77). Participants were classified as “highly adherent” if they reported taking 100% of prescribed doses, and “nonadherent” if they had taken ≤80% of prescribed doses (77). Twenty-seven patients were highly adherent, and 13 were nonadherent (77). Authors found that genotypic resistance mutations were found in the majority of participants, despite the fact that self-reported adherence to therapy was good (77).
Nonadherence and sexual risk behaviours
Perhaps the most obvious outcome of nonadherence to ART is onward transmission of HIV; indeed, preventing transmission is one of the main goals of ART (78). HIV treatment reduces a person’s viral load to an undetectable level, and people with HIV who maintain an undetectable viral load have effectively no risk of transmitting HIV to their HIV-negative partners through sex (78). Thus, nonadherence to ART “…undermines the potential for antiretroviral therapies to reduce HIV transmission” (79).
One study from 2010 among males living with HIV sought to determine if nonadherence compared to adherence to ART was associated with HIV transmission risks (80). Participants included 226 men living with HIV recruited from AIDS service organizations, health care providers, social service agencies, and infectious disease clinics in Atlanta, Georgia (80). Data was collected between 2008–2009 (80). Adherence to ART was monitored by telephone-based unannounced pill counts, and data regarding sexual risk (specifically, vaginal and anal intercourse), sexually transmitted infections, infectiousness beliefs, and substance use was collected using audio-computer assisted structured interviews (80). Authors compared individuals who had <80% adherence to ART to those who were >80% adherent (80). Results found that 26% (n=58) of participants reported <80% adherent to ART (80). The majority of the sample reported being sexually active, with nearly one in three participants reporting at least one sex partner who was not living with HIV, in the past three months (80). Compared to those who were <80% adherent to ART, participants who were at least 80% adherent to ART were significantly less likely to demonstrate problematic substance use (80). Authors found that men who were not adherent to ART displayed higher rates of sexual risk behaviours, including a greater number of sex partners and engaging in both unprotected and protected anal intercourse, an association not moderated by substance use (80).
Another study (2014) supports this finding. Participants (n=459) were drawn from five studies of electronically monitored ART adherence that collected data on the frequency of unprotected sexual intercourse (81). The five studies were conducted in Kansas City, Miami, New York, San Francisco and Seattle, and participants were recruited from a wide range of venues: clinics, hospitals, homeless shelters, AIDS service organizations, and community services programs (81). Of note, these five studies were drawn from a larger sample of 16 studies of electronically monitored ART adherence, conducted between 1997–2009 (81). Across all five studies, sexual risk was measured by the number of instances of unprotected vaginal or anal intercourse within a given time frame; as this was different in all five studies, values were adjusted to reflect how often unprotected intercourse occurred per month assessed (81). Pill bottles equipped with a microchip in the cap, which recorded the date and time the pill bottle was opened, was used to measure adherence (81). For these studies, adherence was measured as the proportion of prescribed pills taken in one month (81). In the sample, participants were divided into four groups: gay/bisexual men, heterosexual men, heterosexual women, and lesbian/bisexual women (81). Authors found that heterosexual men living with HIV who were having difficulty adhering to ART were more likely to engage in high-risk sexual behaviours, whereas no such association was observed for gay/bisexual men or women (81).
A third study from 2018 had different findings when the association between nonadherence to ART and engaging in unsafe sexual practices was examined (82). Participants were from the HIV Outpatient Study, a cohort of individuals living with HIV from nine clinics across six U.S. cities (82). In this cross-sectional study, participants were surveyed between 2007–2014; nonadherence was defined as missing at least one dose of HIV medication within the past three days (82). Other data gathered included substance use, number of sexual partners, HIV status disclosure, and condomless intercourse and partner discordance (82). The final sample included 1,729 individuals, 74% identifying as men who have sex with men; of 1,729 participants, 16.5% (n=285) were categorized as nonadherent (82). Of these 285, 42.1% (n=120) reported any condomless sex, and 50.0% (n=122) reported disclosure of HIV status to none or some partners (82). While authors found that nonadherence was significantly associated with having a detectable viral load, nonadherence was not found to be associated with the likelihood of engaging in condomless sex (82). A study from Germany from 2017 among men who have sex with men living with HIV (n=233) found that adherence problems were associated with increased risk of HIV transmission (83). Participants were surveyed on their health status, sexual behaviour, substance use, depression, compulsive sexual behaviour, and sensation seeking; data from time of ART initiation to 2014 was collected (83). Adherence problems were defined as a documented medical assessment that showed a measurable HIV viral load, development of drug resistance, and/or low drug levels (83). Participants were divided into three groups: a low transmission risk group (those who persistently achieved a viral load below the detection threshold), a high-risk transmission group (those with a measurable viral load, who are ART-naïve participants or those during a treatment-free interval with a low likelihood of transmitting resistant HIV strains), and a second high-risk transmission group (those with a measurable viral load who can potentially transmit ART resistance) (83). Compared to the low transmission risk group, those in the second high risk transmission group were significantly more likely to have adherence problems (OR 27.90; 95% CI 3.44–226.17, p=0.002) (83).
Factors that may impact local applicability
This review summarizes some of the population-level outcomes associated with nonadherence to ART. A few important caveats should be considered when interpreting the findings of this review: the definition of adherence in the literature is varying, as are the means of its monitoring (27); there is no “gold standard” to measure adherence to ART (53, 55); and the studies reporting outcomes to ART nonadherence included in this review used data from a wide range of years, when aspects of ART changed considerably. Furthermore, when “ART” is used in this review, it is used as a general, all-encompassing term referring to antiretroviral treatment that utilizes two or more active agents.
What we did
We searched Medline (including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) using a combination of the term HIV in titles or abstracts AND (MeSH term Antiretroviral Therapy or text term Antiretroviral) AND (adher* or non-adher* or nonadher* in titles or abstracts). Studies from low- and middle-income countries were excluded using Cochrane Effective Practice and Organisation of Care filter based on the World Bank list of countries (2019). Searches were conducted on March 9, 2021 and results limited to English articles published from 2006 to present. Reference lists of identified articles were also searched. Google (grey literature) searches using different combinations of these terms were also conducted. The searches yielded 2,875 references from which 83 were included.
Reference list
- Kangethe A, Polson M, Lord TC, Evangelatos T, Oglesby A. Real-world health plan data analysis: Key trends in medication adherence and overall costs in patients with HIV. Journal of Managed Care & Specialty Pharmacy. 2019;25(1):88–93.
- Pruitt Z, Robst J, Langland-Orban B, Brooks RG. Healthcare costs associated with antiretroviral adherence among Medicaid patients. Applied Health Economics & Health Policy. 2015;13(1):69–80.
- Zhang S, Rust G, Cardarelli K, Felizzola J, Fransua M, Stringer HG, Jr. Adherence to highly active antiretroviral therapy impact on clinical and economic outcomes for Medicaid enrollees with human immunodeficiency virus and hepatitis C coinfection. AIDS Care. 2015;27(7):829–35.
- Gardner EM, Maravi ME, Rietmeijer C, Davidson AJ, Burman WJ. The association of adherence to antiretroviral therapy with healthcare utilization and costs for medical care. Applied Health Economics and Health Policy. 2008;6(2):145–55.
- Munakata J, Benner JS, Becker S, Dezii CM, Hazard EH, Tierce JC. Clinical and economic outcomes of nonadherence to highly active antiretroviral therapy in patients with human immunodeficiency virus. Medical Care. 2006;44(10):893–9.
- Abara WE, Adekeye OA, Xu J, Rust G. Adherence to combination antiretroviral treatment and clinical outcomes in a Medicaid sample of older HIV-infected adults. AIDS Care. 2017;29(4):441–8.
- Fielden SJ, Rusch ML, Yip B, Wood E, Shannon K, Levy AR, et al. Nonadherence increases the risk of hospitalization among HIV-infected antiretroviral naive patients started on HAART. Journal of the International Association of Physicians in AIDS Care. 2008;7(5):238–44.
- Sax PE, Meyers JL, Mugavero M, Davis KL. Adherence to antiretroviral treatment and correlation with risk of hospitalization among commercially insured HIV patients in the United States. PloS one. 2012;7(2):e31591.
- Castillo-Mancilla JR, Cavassini M, Schneider MP, Furrer H, Calmy A, Battegay M, et al. Association of incomplete adherence to antiretroviral therapy with cardiovascular events and mortality in virologically suppressed persons with HIV: The Swiss HIV Cohort Study. Open Forum Infectious Diseases. 2021;8(2):ofab032.
- Glass TR, Sterne JA, Schneider MP, De Geest S, Nicca D, Furrer H, et al. Self-reported nonadherence to antiretroviral therapy as a predictor of viral failure and mortality. AIDS. 2015;29(16):2195–200.
- Kim JH, Psevdos G, Jr., Gonzalez E, Singh S, Kilayko MC, Sharp V. All-cause mortality in hospitalized HIV-infected patients at an acute tertiary care hospital with a comprehensive outpatient HIV care program in New York City in the era of highly active antiretroviral therapy (HAART). Infection. 2013;41(2):545–51.
- Berzosa Sanchez A, Jimenez De Ory S, Frick MA, Menasalvas Ruiz AI, Couceiro JA, Mellado MJ, et al. Mortality in perinatally HIV-infected adolescents after transition to adult care in Spain. Pediatric Infectious Disease Journal. 2020;40(4):347–50.
- Fish R, Judd A, Jungmann E, O’Leary C, Foster C, HIV Young Persons Network. Mortality in perinatally HIV-infected young people in England following transition to adult care: An HIV Young Persons Network (HYPNet) audit. HIV Medicine. 2014;15(4):239-44.
- Nachega JB, Marconi VC, van Zyl GU, Gardner EM, Preiser W, Hong SY, et al. HIV treatment adherence, drug resistance, virologic failure: Evolving concepts. Infectious Disorders — Drug Targets. 2011;11(2):167–74.
- Gardner EM, Hullsiek KH, Telzak EE, Sharma S, Peng G, Burman WJ, et al. Antiretroviral medication adherence and class-specific resistance in a large prospective clinical trial. AIDS. 2010;24(3):395–403.
- Yavuz B, Morgan JL, Showalter L, Horng KR, Dandekar S, Herrera C, et al. Pharmaceutical approaches to HIV treatment and prevention. Advanced Therapeutics. 2018;1(6):1–54.
- Ammassari A, Trotta MP, Shalev N, Marconi P, Antinori A. Beyond virological suppression: The role of adherence in the late HAART era. Antiviral Therapy. 2012;17(5):785–92.
- Dybul M, Fauci AS, Bartlett JG, Kaplan JE, Pau AK. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Recommendations of the Panel on Clinical Practices for Treatment of HIV. Morbidity and Mortality Weekly Report. 2002;51(RR–7):1–55.
- CADTH Common Drug Review. Common drug review new combination product submission: Darunavir/Cobicistat/Emtricitabine/Tenofovir alafenamide (Symtuza): Janssen Canada Inc. 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK540525/pdf/Bookshelf_NBK540525.pdf Accessed March 25, 2021.
- Clay PG, Yuet WC, Moecklinghoff CH, Duchesne I, Tronczynski KL, Shah S, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Research & Therapy. 2018;15(17):1–10.
- Masters MC, Krueger KM, Williams JL, Morrison L, Cohn SE. Beyond one pill, once daily: Current challenges of antiretroviral therapy management in the United States. Expert Review of Clinical Pharmacology. 2019;12(12):1129–43.
- CATIE. Common HIV drugs available in Canada for adults. 2020. Available from: https://www.catie.ca/sites/default/files/common_hiv_drugs_EN_WEB_2020_12_15.pdf Accessed March 26, 2021.
- Altice F, Evuarherhe O, Shina S, Carter G, Beaubrun AC. Adherence to HIV treatment regimens: Systematic literature review and meta-analysis. Patient Preference & Adherence. 2019;13:475–90.
- Ramjan R, Calmy A, Vitoria M, Mills EJ, Hill A, Cooke G, et al. Systematic review and meta-analysis: Patient and programme impact of fixed-dose combination antiretroviral therapy. Tropical Medicine & International Health. 2014;19(5):501-13.
- Nachega JB, Parienti JJ, Uthman OA, Gross R, Dowdy DW, Sax PE, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: A meta-analysis of randomized controlled trials. Clinical Infectious Diseases. 2014;58(9):1297–307.
- Byrd KK, Hou JG, Hazen R, Kirkham H, Suzuki S, Clay PG, et al. Antiretroviral adherence level necessary for HIV viral suppression using real-world data. Journal of Acquired Immune Deficiency Syndromes. 2019;82(3):245–51.
- Carvalho PP, Barroso SM, Coelho HC, Penaforte FRO. Factors associated with antiretroviral therapy adherence in adults: An integrative review of literature. Ciencia & Saude Coletiva. 2019;24(7):2543–55.
- Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdottir TB, Richter C, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: A meta-analysis. BMC Medicine. 2014;12(142):1–14.
- Li H, Marley G, Ma W, Wei C, Lackey M, Ma Q, et al. The role of ARV associated adverse drug reactions in influencing adherence among HIV-infected individuals: A systematic review and qualitative meta-synthesis. AIDS & Behavior. 2017;21(2):341–51.
- Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: A systematic review. AIDS & Behavior. 2012;16(8):2119–43.
- Aidala AA, Wilson MG, Shubert V, Gogolishvili D, Globerman J, Rueda S, et al. Housing status, medical care, and health outcomes among people living with HIV/AIDS: A systematic review. American Journal of Public Health. 2016;106(1):e1–e23.
- Singer AW, Weiser SD, McCoy SI. Does food insecurity undermine adherence to antiretroviral therapy? A systematic review. AIDS & Behavior. 2015;19(8):1510–26.
- Schaecher KL. The importance of treatment adherence in HIV. American Journal of Managed Care. 2013;19(12 Suppl):s231–7.
- Hart JE, Jeon CY, Ivers LC, Behforouz HL, Caldas A, Drobac PC, et al. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: A meta-analysis and systematic review. Journal of Acquired Immune Deficiency Syndromes. 2010;54(2):167–79.
- Binford MC, Kahana SY, Altice FL. A systematic review of antiretroviral adherence interventions for HIV-infected people who use drugs. Current HIV/AIDS Reports. 2012;9(4):287–312.
- Mathes T, Pieper D, Antoine SL, Eikermann M. Adherence-enhancing interventions for highly active antiretroviral therapy in HIV-infected patients — A systematic review. HIV Medicine. 2013;14(10):583–95.
- Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Barnighausen T. Interventions to improve adherence to antiretroviral therapy: A rapid systematic review. AIDS. 2014;28 Suppl 2:S187–204.
- Mbuagbaw L, Sivaramalingam B, Navarro T, Hobson N, Keepanasseril A, Wilczynski NJ, et al. Interventions for enhancing adherence to antiretroviral therapy (ART): A systematic review of high quality studies. AIDS Patient Care & STDs. 2015;29(5):248–66.
- Zuge SS, Paula CC, Padoin SMM. Effectiveness of interventions for adherence to antiretroviral therapy in adults with HIV: A systematic review. Revista Da Escola de Enfermagem Da Usp. 2020;54:e03627.
- Musayon-Oblitas Y, Carcamo C, Gimbel S. Counseling for improving adherence to antiretroviral treatment: A systematic review. AIDS Care. 2019;31(1):4–13.
- Pellowski JA, Price DM, Harrison AD, Tuthill EL, Myer L, Operario D, et al. A systematic review and meta-analysis of antiretroviral therapy (ART) adherence interventions for women living with HIV. AIDS & Behavior. 2019;23(8):1998–2013.
- Rooks-Peck CR, Wichser ME, Adegbite AH, DeLuca JB, Barham T, Ross LW, et al. Analysis of systematic reviews of medication adherence interventions for persons with HIV, 1996–2017. AIDS Patient Care & STDs. 2019;33(12):528–37.
- Hanhoff N, Vu Q, Lang R, Gill MJ. Impact of three decades of antiretroviral therapy in a longitudinal population cohort study. Antiviral Therapy. 2019;24(3):153–65.
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PloS One. 2013;8(12):e81355.
- Montaner JS, Lima VD, Harrigan PR, Lourenco L, Yip B, Nosyk B, et al. Expansion of HAART coverage is associated with sustained decreases in HIV/AIDS morbidity, mortality and HIV transmission: The “HIV Treatment as Prevention” experience in a Canadian setting. PloS One. 2014;9(2):e87872.
- Ortego C, Huedo-Medina TB, Llorca J, Sevilla L, Santos P, Rodriguez E, et al. Adherence to highly active antiretroviral therapy (HAART): A meta-analysis. AIDS & Behavior. 2011;15(7):1381–96.
- University of Calgary: Centre for Health Informatics Alberta Real-World Evidence Consortium. Retrospective database analysis to estimate adherence rates in PLHIV in Canada. 2020. Available from: https://cumming.ucalgary.ca/sites/default/files/teams/30/publications/Parexel%20_Canada_HIVAdherence_Report_Final_05DEC2020.pdf Accessed April 1, 2021.
- Ontario Advisory Committee on HIV/AIDS. Focusing our efforts: Changing the course of HIV prevention, engagement and care cascade in Ontario. 2016. Available from: https://www.health.gov.on.ca/en/pro/programs/hivaids/docs/oach_strategy_2026.pdf Accessed April 1, 2021.
- Arkell C. Getting to undetectable: Population differences in Canada. 2017. Available from: https://catie.ca/en/pif/fall-2017/getting-undetectable-population-inequities-canada#footnote13_x6ej1tj Accessed April 1, 2021.
- Maggiolo F, Di Filippo E, Comi L, Callegaro A, Colombo GL, Di Matteo S, et al. Reduced adherence to antiretroviral therapy is associated with residual low-level viremia. Pragmatic & Observational Research. 2017;8:91–7.
- Wood E, Hogg RS, Yip B, Harrigan PR, O’Shaughnessy MV, Montaner JS. The impact of adherence on CD4 cell count responses among HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes. 2004;35(3):261–8.
- Parrish RG. Measuring population health outcomes. Preventing Chronic Disease. 2010;7(4):1–11.
- Glass T, Cavassini M. Asking about adherence — From flipping the coin to strong evidence. Swiss Medical Weekly. 2014;144(w14016):1–7.
- Office of AIDS Research, National Institutes of Health. HIV treatment adherence. 2021. Available from https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-treatment-adherence Accessed April 8, 2021.
- Castillo-Mancilla JR. Adherence to antiretroviral therapy and pre-exposure prophylaxis: TARGETing the ideal measure. Clinical Infectious Diseases. 2020;70(10):2152–4.
- Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: Challenges, triumphs and the promise of the future. British Journal of Clinical Pharmacology. 2015;79(2):182–94.
- Office of AIDS Research, National Institutes of Health. FDA-approved HIV medicines. 2021. Available from: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/fda-approved-hiv-medicines Accessed April 7, 2021.
- Farr AM, Johnston SS, Ritchings C, Brouillette M, Rosenblatt L. Persistence, adherence, and all-cause healthcare costs in atazanavir- and darunavir-treated patients with human immunodeficiency virus in a real-world setting. Journal of Medical Economics. 2016;19(4):386–96.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behavioral Research. 2011;46(3):399–424.
- Schilling C, Kaye-Blake B, Allen J, Zeng L. The economic impact of nonadherence: Microsimulation modelling for HIV/AIDS in NZ. 2012. Available from: https://nzier.org.nz/static/media/filer_public/9b/c8/9bc85a12-f123-477a-b1ad-02f844ff0b7f/nzier_-_economic_impact_of_non-adherence_nzae_paper_1.pdf Accessed April 14, 2021.
- Statistics Canada. Microsimulation. 2015. Available from: https://www.statcan.gc.ca/eng/microsimulation/index Accessed April 14, 2021.
- Barnett PG, Chow A, Joyce VR, Bayoumi AM, Griffin SC, Nosyk B, et al. Determinants of the cost of health services used by veterans with HIV. Medical Care. 2011;49(9):848–56.
- Dixon T, Hiligsmann M, John D, Ghaleb O, Ahdab A, Li H. Chapter 18 — Pharmacoeconomic analyses and modeling. In: Clinical pharmacy education, practice and research. Cambridge: Elsevier. 2019.
- Boussari O, Subtil F, Genolini C, Bastard M, Iwaz J, Fonton N, et al. Impact of variability in adherence to HIV antiretroviral therapy on the immunovirological response and mortality. BMC Medical Research Methodology. 2015;15(1):1–8.
- Grant C, Bergin C, O’Connell S, Cotter J, Ní Cheallaigh C. High-cost, high-need users of acute unscheduled HIV care: A cross-sectional study. Open Forum Infectious Diseases. 2020;7(2):ofaa037.
- Nijhawan AE, Kitchell E, Etherton SS, Duarte P, Halm EA, Jain MK. Half of 30-day hospital readmissions among HIV-Infected patients are potentially preventable. AIDS Patient Care & STDs. 2015;29(9):465–73.
- Yusuf H, Agwu A. Adolescents and young adults with early acquired HIV infection in the United States: Unique challenges in treatment and secondary prevention. Expert Review of Antiinfective Therapy. 2020:1–15.
- World Health Organization. WHO HIV drug resistance report 2012. 2012. Available from: https://apps.who.int/iris/bitstream/handle/10665/75183/9789241503938_eng.pdf Accessed April 9, 2021.
- Cadosch D, Bonhoeffer S, Kouyos R. Assessing the impact of adherence to anti-retroviral therapy on treatment failure and resistance evolution in HIV. Journal of the Royal Society Interface. 2012;9(74):2309–20.
- Li JZ, Paredes R, Ribaudo HJ, Svarovskaia ES, Metzner KJ, Kozal MJ, et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: A systematic review and pooled analysis. JAMA. 2011;305(13):1327–35.
- Office of AIDS Research, National Institutes of Health. HIV/AIDS glossary: Drug class. 2021. Available from: https://clinicalinfo.hiv.gov/en/glossary/drug-class Accessed April 7, 2021.
- Cevik M, Orkin C, Sax PE. Emergent resistance to dolutegravir among INSTI-naive patients on first-line or second-line antiretroviral therapy: A review of published cases. Open Forum Infectious Diseases. 2020;7(6):1–4.
- Tinago W, O’Halloran JA, O’Halloran RM, Macken A, Lambert JS, Sheehan GJ, et al. Characterization of associations and development of atazanavir resistance after unplanned treatment interruptions. HIV Medicine. 2014;15(4):224–32.
- Rocheleau G, Brumme CJ, Shoveller J, Lima VD, Harrigan PR. Longitudinal trends of HIV drug resistance in a large Canadian cohort, 1996–2016. Clinical Microbiology & Infection. 2018;24(2):185–91.
- Boyd MA, Moore CL, Molina JM, Wood R, Madero JS, Wolff M, et al. Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: An exploratory analysis. The Lancet HIV. 2015;2(2):e42–51.
- von Wyl V, Klimkait T, Yerly S, Nicca D, Furrer H, Cavassini M, et al. Adherence as a predictor of the development of class-specific resistance mutations: The Swiss HIV Cohort Study. PLoS ONE. 2013;8(10):e77691.
- d’Ettorre G, Forcina G, Ceccarelli G, Andreotti M, Andreoni C, Rizza C, et al. Adherence and genotypic drug resistance mutations in HIV-1-infected patients failing current antiretroviral therapy. Journal of Chemotherapy. 2011;23(1):24–7.
- National Institutes of Health, Office of AIDS Research. HIV Treatment: The Basics. 2020. Available from: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/hiv-treatment-basics Accessed April 13, 2021.
- Kalichman SC, Cherry C, White D, Jones M, Grebler T, Kalichman MO, et al. Sexual HIV transmission and antiretroviral therapy: a prospective cohort study of behavioral risk factors among men and women living with HIV/AIDS. Annals of Behavioral Medicine. 2011;42(1):111–9.
- Kalichman SC, Cherry C, Amaral CM, Swetzes C, Eaton L, Macy R, et al. Adherence to antiretroviral therapy and HIV transmission risks: Implications for test-and-treat approaches to HIV prevention. AIDS Patient Care & STDs. 2010;24(5):271–7.
- Remien RH, Dolezal C, Wagner GJ, Goggin K, Wilson IB, Gross R, et al. The association between poor antiretroviral adherence and unsafe sex: Differences by gender and sexual orientation and implications for scale-up of treatment as prevention. AIDS & Behavior. 2014;18(8):1541–7.
- Durham MD, Hart R, Buchacz K, Hammer J, Young B, Yang D, et al. Antiretroviral nonadherence and condomless sex in the HIV Outpatient Study, USA, 2007–2014. International Journal of STD & AIDS. 2018;29(2):147–56.
- Esser S, Krotzek J, Dirks H, Scherbaum N, Schadendorf D. Sexual risk behavior, sexually transmitted infections, and HIV transmission risks in HIV-positive men who have sex with men (MSM) — Approaches for medical prevention. Journal der Deutschen Dermatologischen Gesellschaft. 2017;15(4):421–8.
Suggested citation
Rapid Response Service. Impact of nonadherence to antiretroviral therapy (ART) on population-level health outcomes. Toronto, ON: The Ontario HIV Treatment Network; April 2021.
Prepared by
Danielle Giliauskas and David Gogolishvili
Photo credit
Image adapted from the Office of AIDS Research and the National Institutes of Health