Questions
- What are the best practices (including successes and challenges) of immediate initiation of ART after HIV testing and diagnosis?
Key take-home messages
- The benefits of beginning antiretroviral therapy (ART) soon after HIV diagnosis include improved health outcomes across a wide variety of settings (1–5) as well as decreased long term healthcare costs (6, 7).
- Generally, studies found that time to virologic suppression decreased when ART was initiated immediately, when compared to historical cohorts or those receiving deferred HIV treatment (8–16).
- To facilitate an option of immediate ART, additional resources may be required; this includes added appointment slots (14), training and education for staff (9, 12, 13), a dedicated medical provider (13), a dedicated patient navigator (17), and more human resources in general (18).
- Ideal regimens for early ART initiation should: be coformulated as a single tablet, be well-tolerated, have few drug interactions, have a high barrier to resistance, and be effective among individuals who present with a low CD4 count and high viral load (19).
The issue and why it’s important
Current HIV care guidelines in high-income settings recommend that individuals who test positive for HIV should initiate antiretroviral therapy as soon as possible (20–22). While the time to develop clinical manifestations and disease progression can vary among individuals initially infected with HIV (23), studies have found that initiating ART as soon as possible after a positive HIV diagnosis can lead to improved clinical outcomes (4), even among older adults (5). A 2018 meta-analysis by Song et al. included 12 studies from a variety of settings and found that among people living with HIV, the risk of mortality and developing AIDS was reduced with early initiation of ART (2). In 2019, a review published in the Cochrane Database of Systematic Reviews identified seven randomized controlled trials among 18,011 adults in low- and middle-income settings and found that initiating ART within one week of diagnosis improved outcomes across the HIV care cascade (1).
The benefits of initiating HIV treatment in early asymptomatic HIV infection have been demonstrated by the START trial (3). Between 2009–2013, 4,685 individuals living with HIV who had a CD4 count greater than 500 cells/µL were randomized to one of two groups: an immediate-initiation group (n=2,326), or a deferred-initiation group (n=2,359); participants were from 215 sites in 35 countries (3). Individuals in the deferred-initiation group were to begin treatment only when their CD4 count decreased to 350 cells/µL, if an AIDS-related event occurred, or if a condition dictated the use of ART (e.g. pregnancy) (3). In 2015, on the basis of an interim analysis, the study’s independent data and safety monitoring board “…recommended that individuals in the deferred-initiation group be offered antiretroviral therapy” (3). That is to say, authors identified a net benefit when ART was initiated in adults living with HIV who had a CD4 count greater than 500 cells/µL, compared to deferring ART until the CD4 count had declined to 350 cells/µL (3).
Secondary analysis from the START trial found that immediate initiation of ART appeared to reduce the risk of multiple bacterial infections (24) and significantly reduced the risk of infection-related cancer (25). Additionally, a 2019 analysis from the START trial found that those recently infected with HIV (i.e. ≤6 months) who were randomized to the immediate-initiation group had the greatest increase in the CD4 cell count (26). However, a separate analysis found that immediate initiate of ART in START participants resulted in a greater decline of bone mineral density in the hip and spine (27).
In addition to the health benefits of early initiation of ART, two U.S. studies have identified that starting ART early is associated with cost benefits. In a study of individuals living with HIV on Medicaid, authors found that individuals who initiated ART within 14 days of HIV diagnosis accumulated lower total health care costs compared to those who delayed treatment in the 36-month period following diagnosis (7). Another study among individuals with commercial insurance coverage living with HIV in the U.S. found that those who initiated ART within seven days of treatment had lower total health care costs compared to those who started ART between eight and 60 days after diagnosis (6). Additionally, an analysis in the UK found that it may be a cost-effective strategy to increase screening for HIV and initiate ART early (28).
Despite the benefits of initiating ART immediately, there are several reasons why initiating ART after being diagnosed with HIV may be delayed. Immediate initiation of ART is in contrast to a “patient readiness” model of ART, where a patient’s support system, coping skills, and HIV knowledge are considered before ART is started (29, 30). In a review of qualitative research among people living with HIV in high-income settings, authors found that initiating treatment was a “…complicated process involving careful deliberation of the personal risks and benefits of treatment within the broader contexts of everyday life” (31). Some studies have identified why individuals may delay HIV treatment, including: prioritization of post-test counselling, education, unstable housing, and substance use issues (8); proof of readiness to start ART, confirmed by attending multiple appointments (8); and a prolonged personal adjustment period to receiving a positive HIV diagnosis (32). This review examines models that facilitate ART initiation as soon as a positive HIV diagnosis is received, describing the processes and outcomes at a variety of sites offering this service.
What we found
There are multiple studies that describe an option of ART initiation that is “immediate”, “rapid”, or available “as soon as possible”. One author suggests that “rapid” initiation of ART typically refers to treatment that starts within days or weeks (ideally two weeks) of diagnosis and that “immediate” initiation refers to starting ART on the day of diagnosis (33). However, there does not appear to be a general consensus that these terms refer to the elapse of a specific amount of time between diagnosis of HIV and initiation of ART. Rather, it appears that ART that is “immediate”, “rapid”, or available “as soon as possible” can refer to starting ART treatment on the same day HIV is diagnosed, or within a time frame of mere hours, to up to two weeks after diagnosis. Despite the variety of timeframes used to describe these initiatives, the majority of the programs we identified aimed to have newly-diagnosed people living with HIV initiate ART within two weeks.
However, there does appear to be consensus that programs offering ART that is “rapid”, “immediate”, or “as soon as possible” refer to initiation of ART before the results of the baseline laboratory assessment are received and assessed (19, 34). This is described in a study by Whitlock et al. (2019) at 56 Dean Street, a sexual health clinic in London, UK, where a “rapid initiation option” for ART treatment was launched (14):
Until July 2016, following a new diagnosis of HIV infection at our service, the standard time until the first appointment with a physician was 14 days, in keeping with British HIV Association (BHIVA) guidance. This was primarily so that the results of the baseline blood tests taken at diagnosis might be available, including CD4 count, HIV viral load, human leucocyte antigen (HLA)-B5701 genotype and genotypic viral resistance testing results, thereby facilitating discussion and implementation of an appropriate antiretroviral treatment (ART) regimen.
The following sections offer detailed descriptions of ART programs that aim to initiate ART immediately after a positive HIV test result. Unless otherwise specified, when the terms “immediate”, “rapid”, or “as soon as possible” are used in this review, it refers to ART that was initiated before the results of baseline laboratory results were received and assessed.
Models describing immediate initiation of ART
RAPID
In 2013, the San Francisco General Hospital HIV Clinic (Ward 86 HIV Clinic) launched an intervention called RAPID (Rapid ART Program for Individuals with an HIV Diagnosis), which was designed to facilitate same-day treatment to individuals newly diagnosed with HIV (8). Between June 2013 and December 2014, 39 patients with acute HIV infection, recent HIV infection (<6 months), or a CD4 count of less than 200 cells/µL were eligible for RAPID, and were managed according to the RAPID protocol (8). The RAPID intervention had seven components (8):
- Access to an HIV specialty physician or nurse practitioner on day of diagnosis; taxi vouchers were provided for those who needed transportation from a testing site to the clinic.
- This same-day visit lasted 3–4 hours, and consisted of HIV risk-reduction education and benefits of ART; contraindications to ART were assessed, and baseline laboratory tests were ordered.
- The insurance pre-approval process was accelerated, and follow-up of insurance applications were prioritized.
- Pre-approved ART regimens (approved by a local expert committee who accounted for patterns of transmitted drug resistance and toxicity) were used.
- Each pre-approved ART regimen was available as a 5-day started pack (if needed) while insurance was arranged.
- Participants who accepted ART were offered their first dose in the clinic, with the provider in the room for support.
- Nurses from RAPID contacted participants within seven days to review laboratory test results, inquire about adherence, discuss pharmacy or prescription issues, and ask about medication side effects.
To assess if RAPID impacted the acceptability, safety, and short-term efficacy of ART, authors considered all patients with new HIV infection who were referred to the clinic between June 2013 and December 2014; some were managed by the RAPID intervention (n=39) or by the clinic’s standard of care (n=47) (8). While both groups had similar demographic characteristics, CD4 cell counts, and viral load distributions, the RAPID group did have a greater number of individuals with acute or recent HIV infection (8). The most common RAPID regimen initially prescribed was tenofovir disoproxil fumarate/emtricitabine plus dolutegravir (n=26; 66.7%) (8). This was followed by tenofovir/emtricitabine/elvitegravir/cobicistat (n=7; 17.9%), tenofovir disoproxil fumarate/emtricitabine plus dolutegravir plus ritonavir (n=4; 10.3%), tenofovir disoproxil fumarate/emtricitabine plus raltegravir (n=1; 2.6%), and abacavir plus lamivudine plus dolutegravir (n=1; 2.6%) (8).
Table 1 presents the median time in days it took to reach clinical milestones for each group, from the date of clinic referral. In parentheses beside the median number of days is the spread between the first and third quartile of data (i.e. the middle range of the data set), referred to as the interquartile range (IQR). Note that the median number of days to reach each clinical milestone was lower for those in the RAPID intervention.
Table 1. Time to achievement of clinical milestones (8)
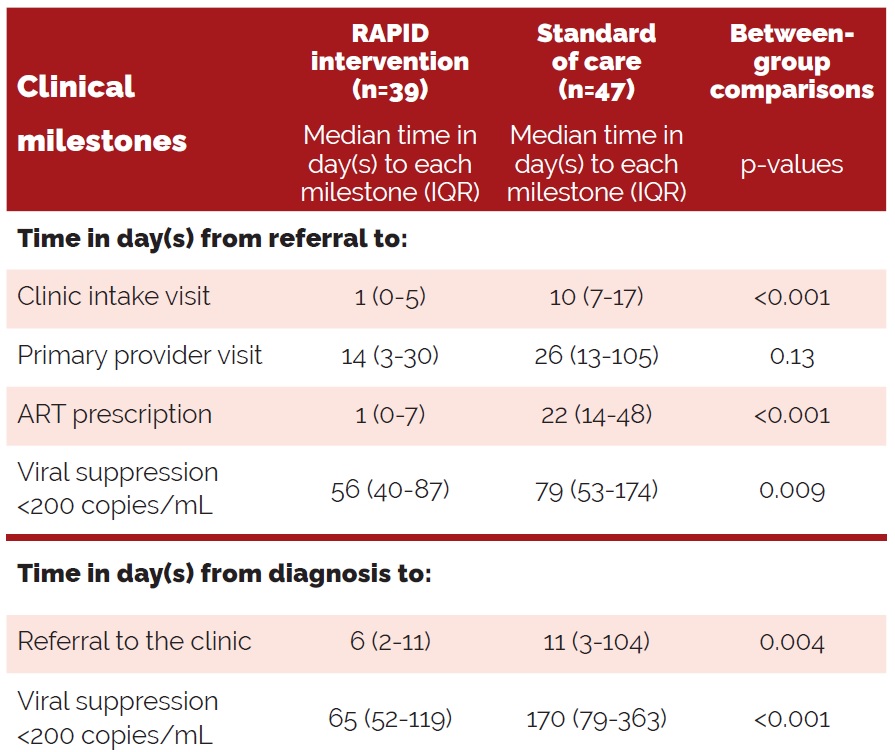
In terms of acceptability, authors report that 35 of the 39 patients (90%) in the RAPID cohort took their first dose at the clinic; and 37 (95%) had started ART within 24 hours of their clinic visit, (8). As for safety, ART regimen modifications were more frequent among those in RAPID: two individuals changed regimens due to a rash, and ten changed to a single-pill regimen containing abacavir once it was determined that the individual did not carry the H-LAB*57:01 allele (8), as individuals who test positive for H-LAB*57:01 are at an increased risk of hypersensitivity when taking an abacavir-containing regimen (35). No changes to ART regimens due to virologic failure or drug resistance (once genotyping results were received) were made (8). In 2014, RAPID became the standard of care at the Ward 86 HIV Clinic (10).
In 2015, the San Francisco Getting to Zero (GTZ) initiative prioritized citywide implementation of RAPID (9, 36). The goal of expanding RAPID was to link all new HIV diagnoses to care within five days and initiate ART at the first visit by optimizing existing systems (9). HIV providers at clinical sites reporting high volumes of new HIV diagnoses and sociodemographic disparities (e.g. youths, Latinx, and African Americans) were prioritized for RAPID training (9).
HIV surveillance data from the San Francisco Department of Public Health was used to assess changes in time from diagnosis to three endpoints: linkage to care, initiation of ART, and first virologic suppression (9). Between 2013–2017, 1,354 individuals were newly diagnosed with HIV in San Francisco (9). Several trends were observed between 2013–2017: median time from first care visit to diagnosis decreased from 9 days to 5 (p<0.001); median time from first care visit to ART initiation decreased from 28 days to 1 (p<0.0001); and median time from initiation of ART to viral suppression decreased from 79 days to 50 (p=0.0007) (9). Finally, median time from diagnosis to first viral suppression decreased by 48%, from 145 days to 76 (p<0.0001) (9). Authors note that this improvement is of particular significance as it required improvements at each step of the HIV care cascade (9).
It should also be noted that there were some disparities in RAPID outcomes: Latinx patients were more likely to initiate ART at first visit; transgender women were least likely to initiate ART at first visit; and time to viral suppression varied among African Americans and people who inject drugs (9, 30). These disparities in the context of immediate ART are further discussed in a later section. To determine long-term viral suppression rates among individuals managed according to RAPID, a retrospective chart review of clients enrolled in the RAPID program at Ward 86 HIV Clinic from 2013–2017 was conducted (10). Between 2013–2017, 225 individuals were referred to RAPID; of these, 96.0% (n=216) started ART immediately (10). Of note, at the time of the first visit, 51.4% had a significant substance use disorder, 48.1% had a mental illness diagnosis documented by the International Classification of Diseases (ICD-10) code, and 30.6% were unstably housed or homeless (10). Median follow-up time for the sample was 1.09 years, with a range of 1 month–3.92 years (i.e. the entire spread of the data set) (10). Within one year, 95.8% of the cohort achieved viral suppression of less than 200 copies/mL (10).
JumpstART
In 2018 at the Conference on Retroviruses and Opportunistic infections, preliminary results of the JumpstART Program were presented. This is an initiative in sexual health clinics in New York City, described as immediate, on-site ART at the time of HIV diagnosis, paired with navigation and linkage to care (37). Results were promising, demonstrating that initiating ART at the time of diagnosis was both feasible and acceptable, resulting in higher ART initiation rates (37). A more comprehensive analysis of JumpstART, including the impact on viral load, was published in 2021 and is discussed below (11).
In 2014, in the state of New York, a three-point plan to end the AIDS epidemic (EtE) was released (11, 38). To support EtE efforts, JumpstART was implemented in eight sexual health clinics operated by the New York City Department of Health and Mental Hygiene from November 2016 to August 2019 (11). JumpstART offered patients newly diagnosed with HIV (based on a reactive point of care test) a one-month supply of ART immediately (11). A retrospective analysis of individuals diagnosed at one of these eight sexual health clinics between 2016 and 2018 was conducted to evaluate time to viral suppression and predictors of viral suppression among those who were and were not a part of the JumpstART service (11).
From the total sample of 303 individuals who were diagnosed with HIV, 230 (76%) were classified as JumpstART and 73 (24%) as non-JumpstART (11). Both groups had a similar proportion of individuals with acute HIV infection, and similar CD4 counts at diagnosis; additionally, demographic and behavioural variables were comparable across both groups (11). However, JumpstART patients had more sexual partners in the three months prior to diagnosis, and were older (47.0% vs. 31.5% aged ≥30 years) (11). Among individuals who achieved viral suppression (<200 copies/mL) during follow-up (n=279; 92%), the median number of days to viral suppression among those in the JumpstART cohort was 31 (IQR 24–51); for non-JumpstART participants, it was 95 (IQR 52–153) (11). While 74% of all patients (225 of 303) were virally suppressed within three months, JumpstART patients were significantly more likely than non-JumpstART patients to achieve viral suppression in this timeframe (p<0.0001) (11). Additionally, among individuals linked to care within 30 days (n=197), those in JumpstART were more likely to achieve viral suppression within the first three months after adjusting for age and baseline viral load (adjusted hazard ratio 3.35, 95% confidence interval [CI] 2.24–5.01) (11).
CrescentCare Start Initiative
CrescentCare is a Federally Qualified Health Center in the southern U.S. that partnered with the New Orleans Office of Health Policy to initiate ART within 72 hours of new HIV diagnoses by improving navigation and accelerating clinic intake (12). The program, CrescentCare Start Initiative (CCSI), commenced in December 2016 (12). HIV testing sites and sexually transmitted infections (STI) clinics run by CrescentCare were leveraged, and referral networks were informed (12). A Standard Operating Procedure was developed and disseminated, and all care providers received training (12). Linkage to care for newly diagnosed individuals was managed by a navigator, who was available 24 hours a day (12). Similar to the RAPID, the intake process was streamlined, and the first dose of ART was directly observed (12). Additionally, a medical checklist was developed to standardize workflow (12). Providers were encouraged to prescribe tenofovir alafenanide/emtricitabine plus dolutegravir due to this regimen’s effectiveness and tolerability (12). Note that this is same regimen prescribed to the majority of individuals in the RAPID trial (discussed above).
In 2018, results from the first eight months of the CCSI cohort (n=77) were compared to a historical cohort from the previous year (12). This historical cohort consisted of 29 individuals who tested positive for HIV at various CrescentCare testing sites and were subsequently linked to care (12). At baseline, there were no significant differences between the two cohorts (12). In the historical cohort, mean time to linkage was 30 days (95% CI 25.1–43.6); for the CCSI cohort, the mean time was 1.3 days (95% CI 1.09–1.51) (12). Additionally, mean time to viral suppression (defined as <200 copies/mL) was 68 days (95% CI 60–92) in the historical cohort; and 30 days (95% CI 27–34) in the CCSI cohort (12).
Authors concluded that the CCSI intervention resulted in high rates of linkage to care and shorter time to viral suppression compared to the historical cohort (12). Authors note that this care model was successful due to use of a full-time navigation specialist who was essential for patient engagement, and that inclusion of a Standard Operating Procedures manual and operational checklist was necessary for providers who were not HIV specialists (12).
Another study provided a comparison of two matched cohorts receiving HIV care at CrescentCare (39). The first cohort, CCSI, included individuals who were newly diagnosed with HIV and linked to care within 72 hours of diagnosis (39). The second cohort, Early Intervention Services (EIS), included ART-naïve individuals who were linked to care more than 72 hours after HIV diagnosis (39). All individuals in both CCSI and EI were offered same-day ART and enrolled in the program between December 2016 and February 2018 (39). A continuum of care was developed for both cohorts and compared four measures: linkage to care (i.e. two provider visits, separated by 90 days, in the span of 12 months), immediate initiation of ART, retention in care, and viral suppression (i.e. <200 copies/mL within 6 months) (39).
During the study period, 130 patients were referred to the CCSI cohort; of these, 126 (97%) were linked within 72 hours of diagnosis; the four remaining patients were lost to follow up (39). Thus, 126 CCSI individuals were started on ART immediately (39). At baseline, 20% of participants (n=25) had a mental health diagnosis documented by the ICD-10 code, and 38% (n=48) were diagnosed with a bacterial STI (39). Median CD4 count was 444 cells/µL and median viral load was 42,600 copies/mL at baseline (39). Every patient with the exception of two started on a regimen of tenofovir alafenanide/emtricitabine plus dolutegravir (39).
The EIS cohort consisted of any individual who was linked to care more than 72 hours after HIV diagnosis (n=69) with a median time to care of 27.5 days (39). All participants except for one were prescribed ART the day they were linked to care (39). At baseline, 33% of participants (n=23) had a mental health diagnosis documented by the ICD-10 code, and 46% (n=32) were diagnosed with a bacterial STI (39). Median CD4 count was 271 cells/µL and median viral load was 70,150 copies/mL at baseline (39). All but three patients started on a regimen of tenofovir alafenanide/emtricitabine plus dolutegravir (39).
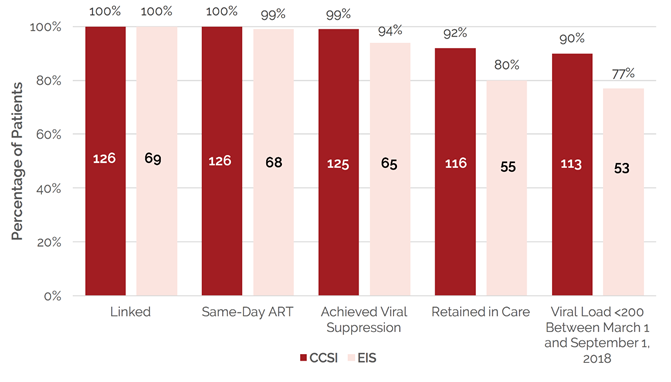
The following graph (Figure 1), adapted from Halperin et al. (2019), illustrates the differences in the continuum of care between the two cohorts (39).
Evidently, individuals in the CCSI intervention had better outcomes across the continuum when compared to the EIS group (39). Authors suggest that the delayed linkage to care in the EIS group led to lower rates of follow-up and suboptimal adherence (39). These differences could also be explained by the “profound relationship” that develops between the provider and patient, as HIV diagnosis, initiation of ART, and counselling all occur on the same day (39).
Figure 1. CCSI/EIS Continuum of care: December 1, 2016–March 1, 2018 (39)
REACH
The Rapid Entry and ART in Clinic for HIV (REACH) program was launched in May of 2016 by the Infectious Diseases Program (IDP) of the Grady Health System, the largest HIV care provider in the U.S. state of Georgia (13). The IDP is an outpatient clinic that serves more than 6,000 patients, most of whom have no insurance or are underinsured (13). The goals of the REACH program were to streamline clinic enrollment, expedite linkage to care, and initiate ART within 72 hours of presentation (13).
A retrospective cohort study was conducted to measure outcomes of the REACH program at IDP (13). Two cohorts were identified: pre-REACH, for those enrolled in care at IDP from January to May 2016, and post-REACH, for enrollees entering care at IDP from May 2016 to July 2016 (13). This pre- and post-REACH group included individuals who were not on ART at time of enrollment, but had a viral load of >200 copies/mL (13). Data collection occurred for six months after enrollment (13). The total number of new enrollees at IDP who were eligible for analysis was 207, 117 in pre-REACH, and 90 in post-REACH (13). Across both cohorts, the majority of participants were men (79.7%, n=165) and identified as African American/Black (90.8%, n=188) (13). Additionally, more than half reported unstable housing (60.9%, n=126) and unemployment (75.8%, n=157) (13). Substance use in the past three months was reported by 44% (n=91), and 26.1% (n=54) had a mental health diagnosis (13). Nearly 60% (n=124) of individuals were ART naïve (13). Median baseline CD4 count was 146 with an IQR of 45–302 cells/μL (13).
The primary analysis compared time to viral suppression between pre- and post-REACH enrolees (13). Authors found that the median time to viral suppression decreased: pre-REACH, time to viral suppression was 77 days (IQR 62–96), and post-REACH, time to viral suppression was 57 days (IQR 41–70) (p=0.0022) (13). Secondary analysis included examining days to first visit and days to ART initiation (13). Median days to first visit among pre-REACH enrollees was 14 (IQR 12–16) compared to 4 (IQR 1–6) among post-REACH (<0.0001); median days to ART initiation was 22 (IQR 13–38) for pre-REACH, and 4 (IQR 2–8) for post-REACH (<0.0001) (13). Authors concluded that rapid initiation of ART was possible among disenfranchised populations in the Southern U.S. (13).
Finally, authors note three resources necessary to sustain a rapid entry among a vulnerable population:
- Peers or navigators to assist clients through the clinic process and with the necessary paperwork;
- Trained staff to help with application for ART via pharmaceutical assistance programs; and
- A medical provider dedicated to individuals starting ART immediately so that current providers who may already be at capacity are not further burdened (13).
Test and Treat Rapid Response Program
In March of 2016, the Florida Department of Health collaborated with the Jackson Memorial Medical Center and the University of Miami Miller School of Medicine to launch a pilot Test and Treat Rapid Response Program (TRRT) (17). The TRRT aimed to initiate ART on the same day of HIV diagnosis, or at a follow-up appointment scheduled no later than seven days after a positive test result (17). The TRRT clinical site — the University of Miami Adult HIV Outpatient Clinic — is the main entry point for individuals to receive HIV care who live in Miami-Dade County (17). The clinic provides HIV care to over 3,000 individuals annually, the majority of whom are from a racial or ethnic minority group (17). Over 40% of the population is uninsured, and 32% are on Medicaid (17). The time to initiation of ART from the receipt of a positive test result is around 49–73 days for standard of care, a timeline that accounts for confirmatory testing, various referrals, and laboratory assessments (17).
In order to expedite engagement and linkage to care so that ART can be initiated no later than seven days from diagnosis, a collaborative team was established to provide guidance for the newly diagnosed individual (17). This team included a Disease Intervention Specialist, a bilingual Patient Navigator, a Case Manager from the South Florida AIDS Network, and a designated Care Provider; authors note that a focused, coordinated effort was necessary to reduce usual barriers to care (17). The process was direct and immediate: program personnel accompanied individuals newly diagnosed with HIV to case management and then to the outpatient clinic (0.6 miles from the testing site) (17). The role of the Patient Navigator is noted as being particularly significant: during the first year of care, this team member maintained constant contact with the client, assisted in appointment adherence, linkage to other care services, and resolved insurance issues (17).
Between March 2016 and February 2017, 41 individuals who had a positive confirmatory HIV test were included in the TRRT program (17). The majority of patients in this cohort were male (76.0%, n=31) and just over half identified as Hispanic/Latino (54.0%, n=22) (17). Median viral load at first blood draw was 23,236 copies/mL (17). Of the 41 individuals, 25 (60.1%) began ART on their first visit, ten (24.4%) 1–8 days after their first visit, and six (14.6%) 8–14 days after their first visit (17). Thirty-seven of the 41 patients initiated ART with a single-tablet regimen of tenofovir alafenamide/emtricitabine/elvitegravir/cobicistat, with no side effects reported in the first three visits (17).
Over the following 12 months, 36 individuals (87.8%) remained in care (17). Of these, 33 (91.7%) achieved a viral load of <200 copies/mL within 70 days; within 12 months, 35 (97.2%) remained virally suppressed (17). This includes four individuals who had initial viral loads between 4,000,000–5,000,000 copies/mL (17). Additionally, upon entering the TRRT program, the nine individuals presenting with AIDS based on CD4 cell count achieved viral suppression (two did end up transferring to another clinic) (17). Authors concluded that immediate linkage to care was feasible, retention in care improved, and that the program had a positive impact on HIV biological markers (17).
X-TLC & acute HIV infection
The Expanding Testing and Linkage to Care (X-TLC) program is a routine, multi-site HIV testing and linkage to care initiative in Chicago (18). A retrospective study examined the feasibility of implementing rapid initiation of ART for individuals with acute HIV infection within the existing X-TLC program (18). In this study, acute HIV was defined by a reactive fourth or fifth generation HIV test, a negative HIV antibody assay, and a positive HIV viral load (24).
In 2016 and 2017, six of the 13 health centres (labelled as sites A through F) with X-TLC identified at least one individual who presented with acute HIV infection (18). Of these six sites, four (sites B, D, E, and F) independently decided to implement rapid initiation of ART and adopted necessary procedures for all new HIV diagnoses or specifically for those presenting with acute HIV infection (18). Sites A and C adhered to standard linkage procedures (i.e. linkage within 30 days) and had no specific timeline for ART initiation; additionally, sites A and C only had one dedicated linkage navigator (18). The other four sites (B, D, E, and F) had two dedicated linkage navigators, plus a designated infectious disease provider who reviewed results, expedited linkage to care, and determined the need for rapid initiation of ART (18). Other notable differences across these four sites included:
- Sites B and E aimed to initiate ART within 72 hours of diagnosis
- Site D sought to have individuals with acute HIV infection initiate ART within seven days
- Site F aimed to link patients with acute HIV infection to care within seven days
- Site D had additional research funds to support clinical trial staff and rapid initiation of ART for those with acute HIV infection
- Sites B, E, and F all used existing staff and procedures to manage ART initiation for those with acute HIV infection (18).
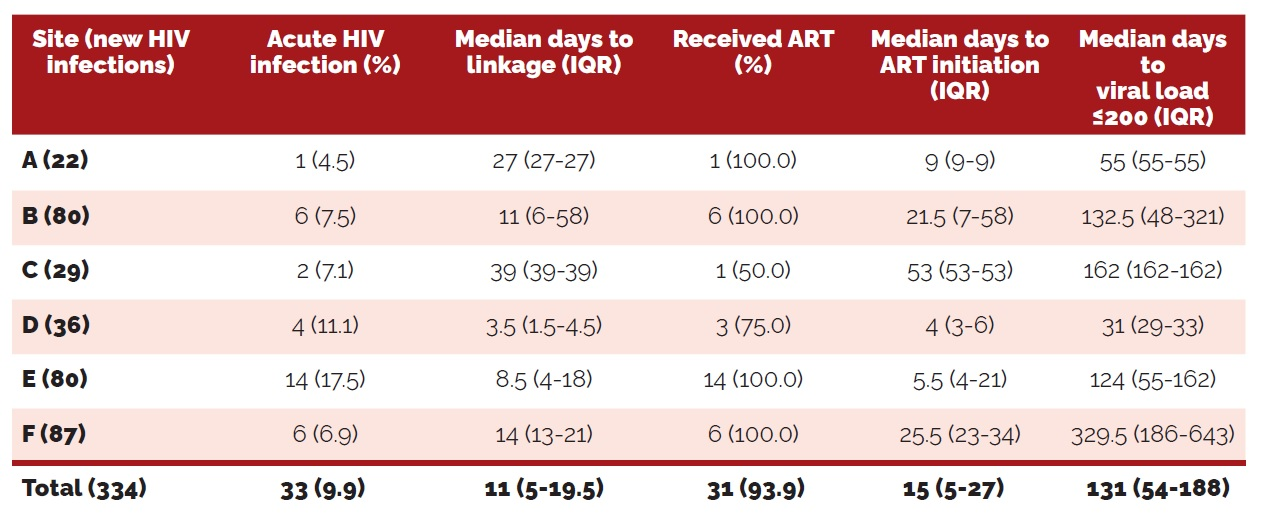
During 2016 and 2017, there were 334 new diagnoses of HIV at sites A–F; 33 (9.9%) of these individuals presented with acute HIV infection (18). Outcomes of the individuals with acute HIV in each grouping is displayed in Table 2 (below), adapted from McNulty et al. (2020).
Evidently, there was considerable variation in HIV care continuum outcomes among sites with the X-TLC program, especially among days to ART initiation and days to viral suppression (18). However, it is important to note that the only site that had clinical trial staff and funding was site D, which demonstrated the shortest amount of days to linkage to care, initiation of ART, and viral suppression (18).
Table 2. Care continuum outcomes for individuals with acute HIV in X-TLC (18)
Open Arms Healthcare Center
Open Arms Healthcare Center (OAHCC) is a community-based clinic in Jackson, Mississippi that began to provide holistic HIV care in 2014, and in 2016 offered same-day initiation of ART (40). A retrospective study was conducted to evaluate those who initiated ART within seven days of diagnosis (Rapid-ART group) and those who did not (Non-Rapid ART group) (40). Between January 2016 and December 2018, 70 newly diagnosed individuals presented for treatment at OAHCC, and 63 were included in the study: 16 in Rapid-ART and 47 in Non-Rapid ART (40). The median time from HIV diagnosis to initiation of ART in the Rapid-ART group was 5.5 days (range 0–7), and in the Non-Rapid ART group, 15 days (range 0–596) (40). Additionally, time from diagnosis to viral suppression (<200 copies/mL) was 55 days (range 26–179) in the Rapid ART group compared to 75 (range 6–44) in the Non-Rapid group (40). However, for initiation of ART to viral suppression, median time was 50 days (range 21–172) in the Rapid-ART group and 46 days (range 21–240) in the Non-Rapid ART group (40).
San Diego Primary Infection Resource Consortium
An observational study from California among individuals newly-diagnosed with HIV enrolled in the San Diego Primary Infection Resource Consortium (SD PIRC) between August 2010 and December 2015 examined the impact of early ART initiation and type of regimen on time to viral suppression (41). The analytic sample comprised of 86 individuals diagnosed with HIV who initiated ART within 30 days of diagnosis (41). Participants were screened using a community-based HIV testing program that provides free, point-of-care rapid HIV testing (41). At the first HIV care intake visit, baseline laboratory tests were ordered and patients were offered immediate, free-of-charge ART (41).
Among the 86 individuals, 98% (n=84) identified as male and 2% (n=2) identified as transgender female (41). Forty two percent (n=36) had acute HIV infection, 31% (n=27) had early HIV infection, and 27% (n=23) had established HIV infection (i.e. duration >170 days) (41). Median time from diagnosis to ART initiation was 13 days (range 0–29); median time from first clinic intake to ART initiation was eight days (IQR 0–28) (41).
At week 12 of ART, those who initiated ART on the same-day of first clinic intake were more likely to be virally suppressed than those who did not (41). Authors concluded that early initiation of ART contributed to faster time to viral suppression, but note that more research is needed to determine other factors that influence acceptance of this model of ART initiation (41).
56 Dean Street (London, UK)
56 Dean Street is a sexual health service in London, England that provides HIV testing and treatment (14). Due to patient demand, in July 2016, the service began to provide a “rapid initiation option” where individuals who were diagnosed with HIV at 56 Dean Street were offered a medical appointment and initiation of ART within two days, regardless of acute or chronic infection (14). Of note, this rapid initiation option was initially only available to individuals presenting with acute HIV infection status; results from a 2017 study observed high uptake of immediate ART and rapid viral suppression from May 2014 to October 2015 (42).
To be able to offer the rapid initiation option to all individuals newly diagnosed with HIV, two additional dedicated physician appointment slots were added per day (14). At this first appointment, individuals were medically assessed, and the benefits and drawbacks (to minimize coercion) of immediate initiation of ART were discussed (14). If immediate ART was accepted, individuals were prescribed a regimen containing co-formulated tenofovir disoproxil fumarate/emtricitabine plus a boosted protease inhibitor (e.g. darunavir) (14).
Case-notes of individuals newly diagnosed with HIV between July 2016 and October 2016 at 56 Dean Street who accepted the rapid initiation option (n=101) were retrospectively reviewed (14). To assess the impact of the rapid initiation option, outcomes were compared to a historical cohort (July 2015–October 2015, n=178) (14). The majority of patients in both cohorts (97%) identified as men who have sex with men (14). In the 2016 cohort, median baseline for CD4 count was 474 cells/µL, compared to 516 cells/µL in the 2015 cohort (p=0.03) (14). Median baseline viral load was 77,000 copies/mL in 2015 compared to 65,000 copies/mL in 2016 (p=0.62) (14).
Table 3 (below) was adapted from Whitlock et al. (2019), and illustrates the difference in outcomes between the two cohorts at 56 Dean Street.
Table 3. Clinical outcomes among clients newly diagnosed with HIV: Standard of care vs rapid initiation option (14)
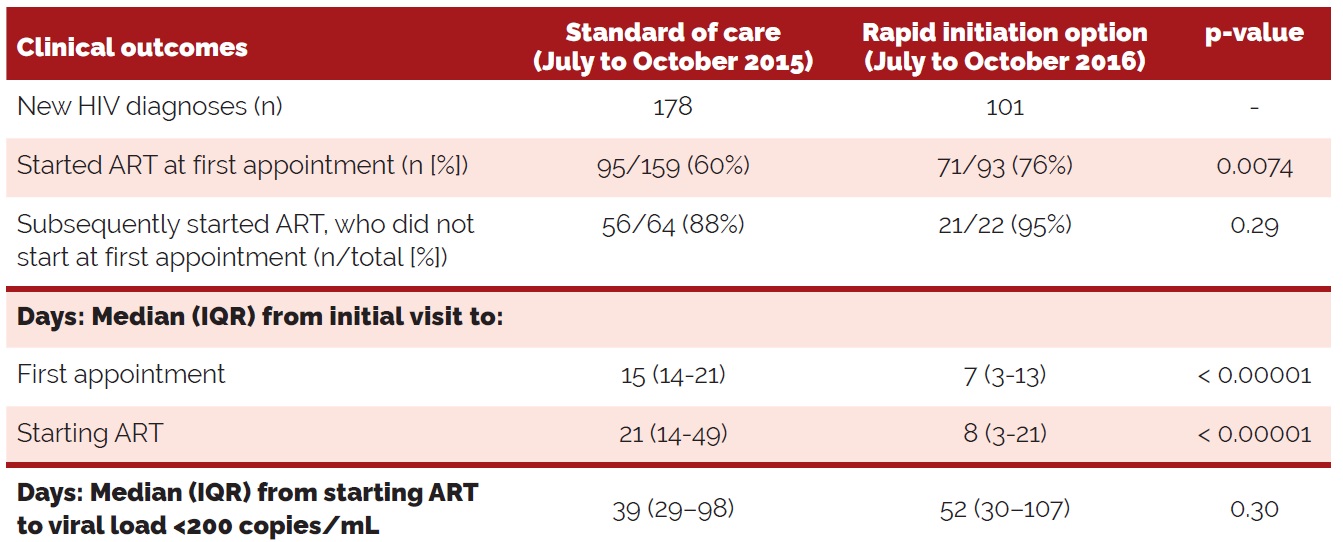
Table 3 illustrates that the rapid initiation option, offered from July to October of 2016, resulted in decreased time from the initial visit to the initiation of ART (14). While authors do note that on average, initiation did not occur within two days of receiving a positive HIV diganosis, those who had acute HIV infection initiated ART within three days of diagnosis (14). Additionally, authors note that of those who subsequently started ART (i.e. not at the first appointment), the proportion who started on ART after the rapid initiation option was introducted was greater (14). Authors also point out that though the time to reach <200 copies/mL was greater in the rapid initiation option cohort, this was not statistically significant (14).
AHI Search and Treat-To-Suppression Strategy (The Netherlands)
In Amsterdam, there is a single large public clinic for STIs that performs more than 50,000 consultations per year (15). Between 2008 and 2017, there were 63,278 HIV testing visits and 1,013 men who have sex with men were diagnosed with HIV (15). Standard of HIV care in this clinic includes confirmation of HIV diagnosis within one week, referral to an HIV treatment centre within five days of diagnosis, and initiation of ART in less than one month (15). In August 2015, this clinic implemented the AHI Search and Treat-To-Suppression Strategy (AHI Strategy) for rapid diagnosis of acute HIV infection, followed by immediate initiation of ART (15). A media campaign was concurrently launched to promote an online intervention aimed at men who have sex with men to increase awareness of acute HIV infection through self-identification of symptoms and increase motivation to get tested at the clinic (15, 43).
A study to determine the feasibility and effectiveness of this strategy was assessed in a 2021, using data from historical cohorts for comparison (15). Of note, the Fiebig HIV staging system (2003) to classify early HIV infection into sequential stages based on testing results was used (44). According to a 2020 publication, three of the five assays used to define the Fiebig staging system are no longer widely used (45). However, the present study does use data from 2002, and thus relies on the Fiebig staging system for classification: acute HIV infection refers to Fiebig stages I–II, early HIV infection refers to stages III–V, and established HIV infection refers to Fiebig stage VI (15).
Authors made several observations from their analysis:
- The proportion of individuals presenting with acute or early HIV infection among positive HIV diagnoses increased from 0.6% (5/876) before implementation of the AHI strategy to 11.0% (15/137) post-AHI strategy
- Before the AHI Strategy, 0.01% of visits (5/47,008) were classified as acute HIV infection; after the AHI Strategy, 0.09% (15/16,270) were classified as acute HIV infection
- Between August 2015–June 2017, median number of hours from intake to receipt of an HIV diagnosis was 6.9 hours (IQR 5.9–7.1) in the standard testing setting; in the AHI strategy, it was 3.5 hours (IQR 1.7–3.8)
- In the AHI Strategy, median number of days from diagnosis to viral suppression (i.e. a viral load of <50 copies/mL) was 55 days (IQR 31–72) compared to 95 days (IQR 63–136) in standard of care for the same time period, and 230 days (IQR 132–480) in a standard of care historical cohort (January 2012–July 2015) (15).
Authors concluded that the AHI Strategy was both feasible and effective as a higher yield of patients with acute HIV infection presented and the time from HIV diagnosis to viral suppression was considerably reduced when compared to standard of care (15).
Immediate referral pathway (British Columbia)
An abstract at the 2015 International AIDS Society describes a pilot intervention in British Columbia where individuals who presented at STI clinics with acute HIV infection were able to choose standard of care or an immediate referral pathway which aimed to established linkage to an HIV specialist within 48 hours (46). Prior to implementation of the pilot, the median number of days in 2013 from diagnosis to linkage was 21.5 days among 45 individuals. From January to September 2014, 19 individuals presented with acute HIV and 16 (84%) chose the immediate referral pathway, which resulted in a median of one day from diagnosis to linkage (46). During that same time period, non-acute HIV diagnoses for standard of care (n=15) was 14.0 days (p<0.05) (46). From qualitative interviews with patients, the immediate referral pathway was highly satisfactory, and providers found that patients were interested in immediate treatment (46).
Immediate ART for youth
A 2021 study describes the results of a rapid start ART approach, introduced on April 2018, at St. Jude’s Children’s Research Hospital (16). This hospital acts as a referral centre for children and youth from birth to the age of 21 living with HIV in a county of Memphis, Tennessee (16). This approach sought to offer ART at the first clinic visit, before receiving results of HIV-related laboratory test results (16).
The baseline cohort consisted of individuals who had their first clinic visit between May 2016 and March 2018 (n=54), and the rapid start cohort which included first visits that occurred between April 2018 and February 2020 (n=70) (16). Cohorts were similar in terms of age, race, sex, CD4 count, and viral load (16). Several comparisons were made between the two cohorts: the number of days from HIV diagnosis to the first clinic visit was shorter in the rapid start cohort compared to the baseline cohort (median 17 days vs. 21, p= 0.045); time from the initial visit to ART initiation was less in the rapid start cohort compared to the baseline cohort (median 0 vs. 14 days, p<0.001); and virologic suppression (<200 copies/mL) was reached sooner in the rapid start cohort compared to the baseline cohort (41 days vs. 54, p=0.01) (16). Authors concluded that starting ART in youth at their first clinic visit was feasible, acceptable, and led to high retention in care (16).
Best practices for rapid initiation of ART
The variety of international studies described above demonstrate the benefits of providing immediate ART in real-world models of care (47). The following sections describes some general considerations when considering implementation of immediate ART.
Drug regimens
The multicenter DIAMOND study in the U.S. evaluated the efficacy, safety, and satisfaction of an oral, single tablet regimen in a rapid-initiation ART care model (34). This study prospectively assessed the initiation of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) among newly diagnosed, treatment-naïve participants, who initiated this single tablet regimen ≤14 days from diagnosis, prior to the availability of laboratory results (34). Upon receipt of laboratory results, individuals who met the stopping criteria discontinued treatment, and transitioned to care apart from the study (34). The study’s main endpoint was the proportion of patients with virologic response at week 48, defined as less than 50 copies/mL (34). Of the 109 participants enrolled in the study, 87% (n=95) were male, 32% (n=35) were Black/African American, and 75% were men who have sex with men (34). At baseline, 25% (n=27) of participants had viral loads ≥100,000 copies/mL, and 21% (n=23) had CD4 cell counts <200 cells/μL (34). By week 48 of the study, 92 (84%) achieved a viral load of <50 copies/mL (34). Of the twelve participants (11%) who prematurely discontinued treatment, none did so due to lack of efficacy or because they developed protocol-defined virologic failure (34). When participation satisfaction was measured at week 48 via the HIV Treatment Satisfaction Questionnaire, 97% of participants reported they were satisfied with their treatment, and 98% reported they would recommend their treatment to other individuals living with HIV (34).
Citing the success of the DIAMOND study, author Garcia-Deltoro (2019) suggests six characteristics for an ideal regimen for early ART initiation:
- Coformulation of a single‑tablet regimen (19). Single tablet regimens, also known as fixed-dose combination treatment, combine at least two active ART agents in a single tablet, taken once daily (48). Compared to multiple-tablet regimens, single tablet regimens have several advantages: therapy continuation, quality of life improvement, viral suppression, cost-effectiveness, and lower health resource utilization (48).
- Well-tolerated (19). In the case of D/C/F/TAF, evidence from the EMERALD and AMBER studies showed that through 48 weeks, this regimen was well-tolerated (19, 49, 50). These findings were further supported by the results of the DIAMOND trial, where only one patient discontinued treatment because of adverse events (19, 34).
- Few drug interactions (19). In the EMERALD, AMBER, and DIAMOND studies, no drug interactions are mentioned (34, 49, 50).
- No prior HLA typing (19). Human leukocyte antigen typing is one of the laboratory tests performed before ART is started to guide initial treatment selection; however, it can take days or weeks to receive these results, thus delaying initiation of ART (19). By initiating ART with a regimen that does not contain abacavir, such as D/C/F/TAF, the need for HLA typing can be avoided (19).
- High barrier to resistance (19). No individuals enrolled in DIAMOND discontinued treatment due to resistance stopping rules (19, 34). Mallolas (2017) notes that “[a]ntiretroviral combinations based on DRV [darunavir] provide a unique robustness in terms of antiviral potency and resistance barrier…” (51). It should also be noted that a 2019 study concluded that immediate initiation of ART increased the risk of drug resistance, but only marginally (52). However, in the HPTN 052 multinational trial, adults in the study arm who initiated ART early had a lower frequency of new drug resistance at virologic failure compared to those in the delayed ART arm, and the main factor associated with reduced drug resistance with early ART was lower baseline viral load (53).
- Efficacy unaltered in patients with low CD4 counts and/or high viral load (19). As noted above, some individuals in the DIAMOND study at baseline had low CD4 counts (24%) and high viral loads (21%); at 48 weeks, 84% of the total sample achieved an RNA level of <50 copies/mL (34).
Health equity
In a 2020 commentary, Mgbako et al. cite the successes of the RAPID model in San Francisco and note that global and U.S. clinical care guidelines are now recommending initiation of ART as soon as possible after HIV diagnosis (30). However, authors also point out that models like RAPID do not explicitly address structural racism, HIV-related stigma, homophobia, and other traumas experienced by Latinxs, African Americans, men who have sex with men, and transgender individuals — all of which can negatively impact outcomes across the HIV care continuum (30). By integrating a health equity approach into programs like RAPID, authors argue that those who are disproportionately impacted by HIV will not be “left behind”, thus avoiding exacerbation of disparities across the HIV care continuum (30). As mentioned previously, the RAPID trial identified some disparities in HIV care continuum outcomes among Latinx individuals, transgender women, African Americans, and people who inject drugs (9, 30). Specifically, authors note that there is a lack of evidence on how individuals among disenfranchised population groups perceive and experience immediate ART, and how immediate ART could potentially impact psychosocial factors (30). A health equity approach to immediate ART would involve a comprehensive understanding of the factors that contribute to disparities at each step of the care continuum (30). Indeed, Boyd et al. (2019) suggest there is a need for health-related quality-of-life data to evaluate how immediate initiation of ART impacts HIV burden and empowerment of people living with HIV (30, 54). Finally, Mgbako et al. call for more qualitative research surrounding immediate ART, which would provide an understanding of “…how vulnerable patients cope with a new HIV diagnosis in the context of iART [immediate ART]” (30).
Factors that may impact local applicability
While all HIV care models discussed in this review were implemented in high-income settings, we only identified one publication from Canada (in British Columbia) that described a model of care that offered “immediate linkage for acutely infected individuals” living with HIV (46). The majority of studies were conducted in the U.S. Additionally, the sociodemographic data collected by the researchers varied: one study identified that the majority of participants were Black (91%), unstably housed (61%), and identified as men who have sex with men (60%); other studies simply noted that the majority of participants identified as men who have sex with with men (13). Furthermore, some studies had a specific focus on individuals presenting with acute HIV (15, 18, 42, 46). Finally, while there was some discussion of resources necessary to establish a program that offers immediate ART, this could widely vary depending on the population which is being served, as well as other factors. Results should be interpreted with caution, as there does not appear to be a “one-size-fits-all” approach to immediate initiation of ART, especially when vulnerable population groups are considered.
What we did
We searched Medline (including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) using a combination of terms (ART or antiretroviral or anti-retroviral or therapy or treatment) in titles or abstracts AND terms (initiati* adj3 (ART or antiretroviral or anti-retroviral or therapy or treatment)) in titles or abstracts AND term HIV in titles or abstracts. Searches were conducted on June 1, 2021 and results limited to English articles published from 2016 to present. Studies from low- and middle-income countries were excluded. Reference lists of identified articles were also searched. Google (grey literature) searches using different combinations of these terms were also conducted. The searches yielded 1,688 references from which 54 were included.
Reference list
- Mateo‐Urdiales A, Johnson S, Smith R, Nachega JB, Eshun‐Wilson I. Rapid initiation of antiretroviral therapy for people living with HIV. Cochrane Database of Systematic Reviews. 2019(6):CD012962.
- Song A, Liu X, Huang X, Meyers K, Oh DY, Hou J, et al. From CD4-based initiation to treating all HIV-infected adults immediately: An evidence-based meta-analysis. Frontiers in Immunology. 2018;9:212.
- The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. New England Journal of Medicine. 2015;373(9):795–807.
- Ford N, Migone C, Calmy A, Kerschberger B, Kanters S, Nsanzimana S, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS. 2018;32(1):17–23.
- Lodi S, Costagliola D, Sabin C, Del Amo J, Logan R, Abgrall S, et al. Effect of immediate initiation of antiretroviral treatment in HIV-positive individuals aged 50 years or older. Journal of Acquired Immune Deficiency Syndromes. 2017;76(3):311–8.
- Benson C, Emond B, Lefebvre P, Lafeuille MH, Cote-Sergent A, Tandon N, et al. Rapid initiation of antiretroviral therapy following diagnosis of human immunodeficiency virus among patients with commercial insurance coverage. Journal of Managed Care & Specialty Pharmacy. 2020;26(2):129–41.
- Benson C, Emond B, Romdhani H, Lefebvre P, Cote-Sergent A, Shohoudi A, et al. Long-term benefits of rapid antiretroviral therapy initiation in reducing medical and overall health care costs among Medicaid-covered patients with human immunodeficiency virus. Journal of Managed Care & Specialty Pharmacy. 2020;26(2):117–28.
- Pilcher CD, Ospina-Norvell C, Dasgupta A, Jones D, Hartogensis W, Torres S, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. Journal of Acquired Immune Deficiency Syndromes. 2017;74(1):44–51.
- Bacon O, Chin J, Cohen SE, Sachdev D, Coffey S, Scheer S, et al. Decreased time from HIV diagnosis to care, ART initiation, and virologic suppression during the citywide RAPID initiative in San Francisco. Clinical Infectious Diseases. 2020;25:25.
- Coffey S, Bacchetti P, Sachdev D, Bacon O, Jones D, Ospina-Norvell C, et al. RAPID antiretroviral therapy: High virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS. 2019;33(5):825–32.
- Pathela P, Jamison K, Braunstein SL, Borges CM, Lazar R, Mikati T, et al. Initiating antiretroviral treatment for newly diagnosed HIV patients in sexual health clinics greatly improves timeliness of viral suppression. AIDS. 2021;10:10.
- Halperin J, Butler I, Conner K, Myers L, Holm P, Bartram L, et al. Linkage and antiretroviral therapy within 72 hours at a federally qualified health center in New Orleans. AIDS Patient Care and STDs. 2018;32(2):39–41.
- Colasanti J, Sumitani J, Mehta CC, Zhang Y, Nguyen ML, Del Rio C, et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the Southern United States. Open Forum Infectious Diseases. 2018;5(6):ofy104.
- Whitlock G, Carbonell M, Blackwell S, Nwokolo N, Dean Street Collaborative Group. Rapid initiation of antiretroviral therapy in those with newly diagnosed HIV infection in London, UK. HIV Medicine. 2019;20(10):699–703.
- Dijkstra M, van Rooijen MS, Hillebregt MM, van Sighem A, Smit C, Hogewoning A, et al. Decreased time to viral suppression after implementation of targeted testing and immediate initiation of treatment of acute HIV infection among men who have sex with men in Amsterdam. Clinical Infectious Diseases. 2020;72:1952–60.
- Patel ND, Dallas RH, Knapp KM, Flynn PM, Gaur AH. Rapid start of antiretroviral therapy in youth diagnosed with HIV infection. Pediatric Infectious Disease Journal. 2021;40(2):147–50.
- Rodriguez AE, Wawrzyniak AJ, Tookes HE, Vidal MG, Soni M, Nwanyanwu R, et al. Implementation of an immediate HIV treatment initiation program in a public/academic medical center in the U.S. South: The Miami Test and Treat Rapid Response Program. AIDS & Behavior. 2019;23(Suppl 3):287–95.
- McNulty M, Schmitt J, Friedman E, Hunt B, Tobin A, Maheswaran AB, et al. Implementing rapid initiation of antiretroviral therapy for acute HIV infection within a routine testing and linkage to care program in Chicago. Journal of the International Association of Providers of AIDS Care. 2020;19:1–7.
- Garcia-Deltoro M. Rapid initiation of antiretroviral therapy after HIV diagnosis. AIDS Reviews. 2019;21(2):55–64.
- British Columbia Centre for Excellence in HIV and AIDS. Therapeutic guidelines for antiretroviral (ARV) treatment of adult HIV infection. 2015. Available from: http://bccfe.ca/sites/default/files/uploads/Guidelines/bccfe-art-guidelines-Oct_14_2015.pdf Accessed June 25, 2021.
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016. Available from: https://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf;jsessionid=4516B6772065A1D89210122D5C5D9384?sequence=1 Accessed June 25, 2021.
- Office of AIDS Research, National Institutes of Health. Recommendations for the use of antiretroviral agents in adults and adolescents with HIV. 2019. Available from: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AA_Recommendations.pdf Accessed June 9, 2021.
- Government of Canada. HIV and AIDS: For health professionals. 2021. Available from: https://www.canada.ca/en/public-health/services/diseases/hiv-aids/health-professionals.html Accessed June 9, 2021.
- O’Connor J, Vjecha MJ, Phillips AN, Angus B, Cooper D, Grinsztejn B, et al. Effect of immediate initiation of antiretroviral therapy on risk of severe bacterial infections in HIV-positive people with CD4 cell counts of more than 500 cells per μL: Secondary outcome results from a randomised controlled trial. Lancet HIV. 2017;4(3):e105–e12.
- Borges AH, Neuhaus J, Babiker AG, Henry K, Jain MK, Palfreeman A, et al. Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clinical Infectious Diseases. 2016;63(12):1668–76.
- Sharma S, Schlusser KE, de la Torre P, Tambussi G, Draenert R, Pinto AN, et al. The benefit of immediate compared with deferred antiretroviral therapy on CD4+ cell count recovery in early HIV infection. AIDS. 2019;33(8):1335–44.
- Hoy JF, Grund B, Roediger M, Schwartz AV, Shepherd J, Avihingsanon A, et al. Immediate initiation of antiretroviral therapy for HIV infection accelerates bone loss relative to referring therapy: Findings from the START Bone Mineral Density substudy, a randomized trial. Journal of Bone & Mineral Research. 2017;32(9):1945–55.
- Brogan AJ, Talbird SE, Davis AE, Wild L, Flanagan D. Is increased screening and early antiretroviral treatment for HIV-1 worth the investment? An analysis of the public health and economic impact of improvement in the UK. HIV Medicine. 2019;20(10):668–80.
- Gebrekristos HT, Mlisana KP, Karim QA. Patients’ readiness to start highly active antiretroviral treatment for HIV. British Medical Journal. 2005;331(7519):772–5.
- Mgbako O, M ES, Olender S, Gordon P, Zucker J, Tross S, et al. Immediate antiretroviral therapy: The need for a health equity approach. International Journal of Environmental Research and Public Health. 2020;17(19):7345.
- Hollingdrake O, Lui CW, Mutch A, Dean J, Howard C, Fitzgerald L. Factors affecting the decision to initiate antiretroviral therapy in the era of treatment-as-prevention: synthesis of evidence from qualitative research in high-income settings. AIDS Care. 2019;31(4):397–402.
- Lee MJ, Venturelli S, McKenna W, Teh J, Negedu O, Florman KE, et al. Reasons for delayed antiretroviral therapy (ART) initiation in the era of early ART initiation guidelines: A retrospective service evaluation. International Journal of STD & AIDS. 2019;30(4):415–8.
- Michienzi SM, Barrios M, Badowski ME. Evidence regarding rapid initiation of antiretroviral therapy in patients living with HIV. Current Infectious Disease Reports. 2021;23(5):7.
- Huhn GD, Crofoot G, Ramgopal M, Gathe J, Bolan R, Luo D, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in a rapid-initiation model of care for human immunodeficiency virus type 1 infection: Primary analysis of the DIAMOND study. Clinical Infectious Diseases. 2020;71(12):3110–7.
- Dean L. Abacavir therapy and HLA-B* 57: 01 genotype. 2018. Available from: https://www.ncbi.nlm.nih.gov/books/NBK315783/pdf/Bookshelf_NBK315783.pdf Accessed June 11, 2021.
- San Francisco Getting to Zero RAPID Committee, San Francisco Department of Public Health. San Francisco program for RAPID ART initiation and linkage to care: Standard operation procedures. 2020. Available from: https://www.gettingtozerosf.org/wp-content/uploads/2020/03/citywide_rapid_protocol_-06MAR20_FINAL.pdf Accessed June 14, 2021.
- Blank S, Borges C, Castro M. Poster 1108. Getting a jump on HIV: Expedited ARV treatment at NYC sexual health clinics. Conference on Retroviruses and Opportunistic Infections. 2017. Available from: https://2jg4quetidw2blbbq2ixwziw-wpengine.netdna-ssl.com/wp-content/uploads/sites/2/posters/2018/1430_Borges_1108.pdf Accessed June 24, 2021.
- New York State Department of Health. Ending the AIDS epidemic in New York State. 2021. Available from: https://www.health.ny.gov/diseases/aids/ending_the_epidemic/ Accessed June 29, 2021.
- Halperin J, Conner K, Butler I, Zeng P, Myers L, Clark R, et al. A care continuum of immediate ART for newly diagnosed patients and patients presenting later to care at a federally qualified health center in New Orleans. Open Forum Infectious Diseases. 2019;6(4):ofz161.
- Gomillia CES, Backus KV, Brock JB, Melvin SC, Parham JJ, Mena LA. Rapid antiretroviral therapy (ART) initiation at a community-based clinic in Jackson, MS. AIDS Research & Therapy. 2020;17(1):60.
- Hoenigl M, Chaillon A, Moore DJ, Morris SR, Mehta SR, Gianella S, et al. Rapid HIV viral load suppression in those initiating antiretroviral therapy at first visit after HIV diagnosis. Scientific Reports. 2016;6:32947.
- Girometti N, Nwokolo N, McOwan A, Whitlock G. Outcomes of acutely HIV-1-infected individuals following rapid antiretroviral therapy initiation. Antiviral Therapy. 2017;22(1):77–80.
- Davidovich U, Dijkstra M, Van Bijnen A, Van Elsen S, Van Der Loeff MS, Verdult F, et al. P4.105 Highly successful engagement in an acute HIV-infection (AHI) awareness campaign and intervention in Amsterdam & its yield of AHI diagnoses at the city’s STI clinic. Sexually Transmitted Infections. 2017;93(Suppl 2):A230.
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17(13):1871–9.
- Facente S, Grebe E, Pilcher C, Busch M, Murphy G, Welte A. Estimated dates of detectable infection (EDDIs) as an improvement upon Fiebig staging for HIV infection dating. Epidemiology & Infection. 2020;148(e53):1–5.
- Thumath M, Moore D, Hull M, Brownrigg B, Sandstra I, Ogilvie G. TUPED 782. Implementation of a rapid referral pathway to HIV treatment for gay men and MSM diagnosed with acute HIV-infection in sexual health clinics in British Columbia. 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention. 2015. Available from: https://www.aidsdatahub.org/sites/default/files/resource/ias-abstract-book-2015.pdf Accessed June 25, 2021.
- Stardust Z, Gray J, McKellar-Stewart N, Cooper C, Positive Life NSW. Evidence brief. Rapid initiation: Models for the immediate uptake of HIV treatment. 2017. Available from: https://apo.org.au/sites/default/files/resource-files/2017-11/apo-nid311880.pdf Accessed July 14, 2021.
- Clay PG, Yuet WC, Moecklinghoff CH, Duchesne I, Tronczyński KL, Shah S, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Research and Therapy. 2018;15(1):1–10.
- Eron JJ, Orkin C, Gallant J, Molina J-M, Negredo E, Antinori A, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naive HIV-1 patients. AIDS. 2018;32(11):1431.
- Orkin C, Molina J-M, Negredo E, Arribas JR, Gathe J, Eron JJ, et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine and tenofovir disoproxil fumarate regimens to single-tablet darunavir, cobicistat, emtricitabine, and tenofovir alafenamide at 48 weeks in adults with virologically suppressed HIV-1 (EMERALD): A phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5(1):e23–e34.
- Mallolas J. Darunavir stands up as preferred HIV protease inhibitor. AIDS Reviews. 2017;19(2):105–12.
- Lodi S, Gunthard HF, Dunn D, Garcia F, Logan R, Jose S, et al. Effect of immediate initiation of antiretroviral treatment on the risk of acquired HIV drug resistance. AIDS. 2018;32(3):327–35.
- Palumbo PJ, Fogel JM, Hudelson SE, Wilson EA, Hart S, Hovind L, et al. HIV drug resistance in adults receiving early vs. delayed antiretroviral therapy: HPTN 052. Journal of Acquired Immune Deficiency Syndromes. 2018;77(5):484–91.
- Boyd M, Boffito M, Castagna A, Estrada V. Rapid initiation of antiretroviral therapy at HIV diagnosis: Definition, process, knowledge gaps. HIV Medicine. 2019;20(Suppl. 1):3–11.
Suggested citation
Rapid Response Service. Immediate initiation of antiretroviral therapy (ART) after HIV diagnosis. Toronto, ON: The Ontario HIV Treatment Network; July 2021.
Prepared by
Danielle Giliauskas and David Gogolishvili
Photo credit
