Questions
- What is the effectiveness of non-occupational HIV post-exposure prophylaxis (PEP)?
- What are the rates of PEP uptake and adherence among people who may have been exposed to HIV?
- What are the best practices of PEP programming and delivery in high-income countries?
Key take-home messages
- HIV post-exposure prophylaxis (PEP) is a safe and effective strategy aimed at preventing infection in those with a recent HIV exposure (1). PEP is for emergency situations and not a substitute for regular use of other HIV prevention strategies (2). PEP is not the right choice for people who may be exposed to HIV frequently (2), and pre-exposure prophylaxis (PrEP) may be better suited to those with ongoing HIV risk (1, 2).
- PEP is typically prescribed as three HIV antiretroviral drugs, started within 72 hours after exposure, and continued for 28 days (1). Recommendations and guidelines are in agreement that 72 hours after exposure is the longest possible timeframe for PEP initiation, and that PEP is unlikely to prevent HIV infection if it is started more than 72 hours after a person is exposed to HIV (3).
- Although recommendations and guidelines agree that “the sooner PEP is started after a possible HIV exposure, the better” (3), there is a difference among jurisdictions regarding the exact timing of PEP initiation within these 72 hours. For example, a much shorter timeframe of two hours (4, 5) is considered “ideal” by the New York State Department of Health (5), and PEP initiation “as soon as possible” after exposure is recommended by the British Columbia Centre for Excellence in HIV/AIDS, the Australasian Society for HIV, the British HIV Association (BHIVA), and others (4, 6–10). According to British HIV Association (BHIVA), PEP should be initiated “preferably” within 24 hours (8, 9, 11). The European AIDS Clinical Society guidelines suggest that PEP should be started ideally <4 hours after the exposure, and no later than 48/72 hours (12).
- While initial patient acceptance of HIV PEP is high, adherence, clinic follow-up, and documented completion rates of PEP vary across studies and population groups (13). PEP adherence and documented completion appear to be low among people who experienced sexual assault (14–17).
- Having high-risk sexual behaviours and a history of sexually transmitted infections are associated with higher PEP uptake, whereas insufficient knowledge, underestimated risk of exposure to HIV, social stigma, and other factors might hinder PEP uptake among men who have sex with men (13).
- Seroconversions among high-risk men who have sex with men who had used PEP in the past suggest that other prevention strategies such as PrEP are needed for this population group (13).
- PEP delivery strategies implemented in different settings include: post-exposure prophylaxis-in-pocket—PIP (providing people with infrequent high-risk HIV exposures a 28-day prescription for PEP before an exposure occurs) (18, 19), and advance provision of self-start home packs (“HOME PEPSE”, a 5-day starter pack for men who have sex with men to self-initiate PEP to reduce time to first dose following HIV exposure) (20).
- Providing PEP starter packs (a 3- to 7-day supply of PEP medications at initial presentation to health care before the full 28-day prescription is provided at a subsequent visit) are used in some jurisdictions and settings (4, 5), but there is evidence suggesting that these starter packs may not improve adherence to PEP and may result in lower adherence and completion rates (21).
- The literature suggests a need for health care providers’ support and capacity building to ensure effective PEP assessment and its optimal use (22–24).
The issue and why it’s important
HIV post-exposure prophylaxis, also known as PEP, is a way to prevent HIV in an HIV-negative person who may have been recently exposed to the virus (25). From a clinical viewpoint, PEP management involves addressing five key questions (26):
- Did an HIV exposure occur?
- If a confirmed or potential HIV exposure occurred, what is the risk of HIV transmission?
- Should this patient initiate PEP and if so, with what drugs?
- What other infectious and non-infectious disease issues should be addressed?
- What is an appropriate follow-up strategy? (26)
According to the 2017 Canadian guideline, non-occupational PEP can be initiated when there is greater than a negligible-to-low risk for HIV acquisition (26), or in other words, after an exposure that is moderate or high risk for HIV transmission (27). If a moderate- or high-risk exposure occurred to a person with a substantial risk of having transmissible HIV, PEP is strongly recommended, but it can also be considered if a moderate- or high-risk exposure occurred with a person who has a low but non-negligible risk of having transmissible HIV (27). PEP is not recommended for individuals who have had a low-risk exposure (such as oral sex), regardless of source HIV status (27). Risk of HIV transmission for receptive anal sex and needle sharing is considered high, for vaginal sex and insertive anal sex—moderate, and for oral sex—low (27). On the other hand, the risk that a person has transmissible HIV infection is considered substantial for those who are viremic (i.e., viral load >40 copies/mL) or whose HIV status is unknown, but they are from a population with high HIV prevalence compared with the general population (e.g., men who have sex with men, people who inject drugs) (27). The risk that a person has transmissible HIV infection is considered low but non-zero for those who are HIV positive and believed to have a viral load <40 copies/mL with concomitant STI present at the time of exposure (27). The risk that a person has transmissible HIV infection is considered “negligible to none” for those who are confirmed HIV negative, or HIV positive with confirmed viral load <40 copies/mL and no known STIs present at time of exposure, and for general population with unknown HIV status (27).
The Public Health Agency of Canada (PHAC) provides a more simplified definition of PEP indications, stating that PEP should be considered for individuals who have had a high-risk exposure to HIV in the workplace (e.g., health care setting) or outside of the workplace (e.g., sexual assault, condomless sex with an HIV-positive partner who is not on treatment or whose viral load is ≥200 copies/ml) (28).
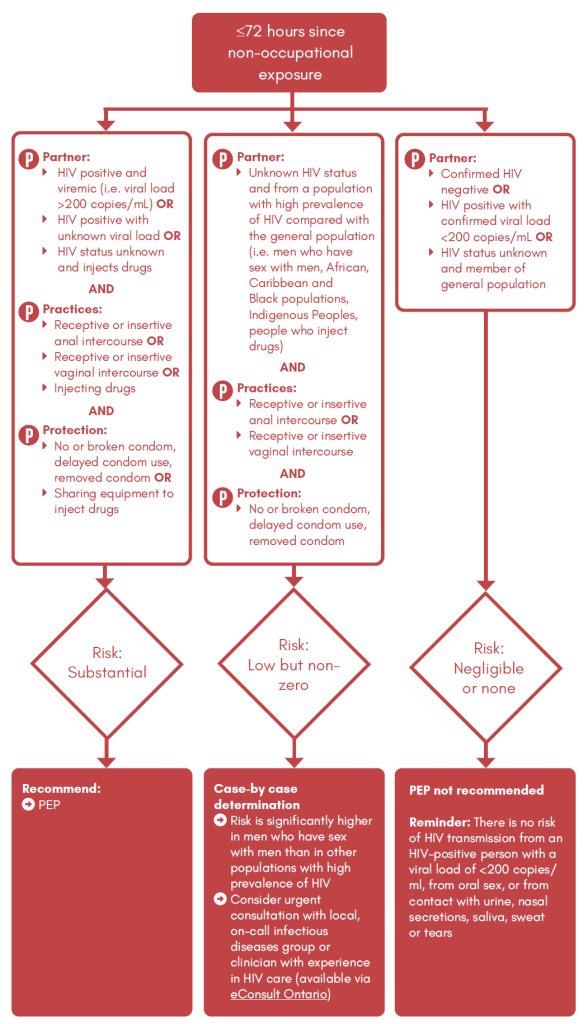
The Ontario guidelines for providers offering HIV testing, published in March 2023, present an algorithm for evaluation of possible PEP treatment for high-risk non-occupational HIV exposures (29). Based on risk practices, partner characteristics, and protection use, the algorithm helps care providers to decide whether PEP use is recommended or if a case-by-case determination should be made (29). This algorithm is presented in Appendix 1.
A full (28-day) course of PEP can cost CAD 900 or more in Canada (25). For example, the cost of a full course of commonly prescribed one-pill-a-day Biktarvy® PEP regimen is up to CAD 1,200 (30). Although occupational PEP is normally covered by workplace insurance, coverage for non-occupational PEP varies across Canadian provinces (25, 31). Additionally, private health insurance plans usually cover the cost of PEP in Canada (25).
This review summarizes evidence on the use of PEP after non-occupational expose (such as sexual exposure or needle sharing during injection drug use). PEP after occupational exposure in health care settings is beyond the scope of this review. We summarize evidence on PEP efficacy, PEP uptake and adherence, and best practices of PEP programming and delivery.
What we found
PEP effectiveness and timing of initiation
PEP is used to help prevent the acquisition of HIV infection by individuals who may have been recently exposed to HIV and it can reduce the risk of HIV infection by more than 80% (28). Generally, PEP is effective when initiated within 72 hours of suspected exposure to HIV and requires antiretroviral medications to be taken once daily for 28 days (28, 32). No randomized, placebo-controlled clinical trial of PEP has been conducted (33). However, data are available from animal transmission models, perinatal clinical trials, observational studies of health care workers receiving prophylaxis after occupational exposures, and observational and case studies of PEP use (33).
Some people in the aforementioned studies acquired HIV despite taking PEP. Many HIV transmissions among people taking PEP occurred because of low adherence (not taking PEP every day for 28 days) and/or ongoing exposures to HIV (25). Effectiveness is likely much higher than 80% if PEP is used consistently and correctly, as prescribed (25). A 2020 systematic review of 74 studies identified 14 studies reporting a total of 500 HIV seroconversions among 19,546 men who have sex with men who had been prescribed PEP, with a seroconversion rate of 2.6% (13). Six of eight studies in this systematic review that reported the interval between PEP initiation and HIV diagnosis indicated that the majority of seroconversions tested HIV-negative at >3 months post-PEP uptake, implying that these seroconversions were unlikely due to PEP failure (13).
The latest systematic review examining PEP use and presented at the Canadian Conference on HIV/AIDS Research (CAHR) in 2023 identified 15 clinical trials and cohort studies published between 2017 and 2022 (34). It found only three cases of seroconversion reported in all 15 studies; one case involved early PEP discontinuation, and two cases involved multiple HIV exposures after starting PEP (34).
Strict adherence to the prescribed regimen is essential for PEP efficacy (28). It is also important to take actions to protect others while taking PEP: always using condoms with sexual partners and not sharing needles, syringes, or other equipment to use drugs (2).
A rapid and effective response to a reported HIV exposure is key to the successful prevention of HIV infection (5). There is some variance among the recommendation on when it is most effective to initiate PEP after exposure, but all guidelines and recommendations agree that PEP should not be initiated beyond 72 hours after exposure.
According to the U.S. Centers for Disease Control and Prevention (CDC), if it is determined that there is substantial risk of HIV transmission and the patient has presented within 72 hours of exposure, HIV non-occupational PEP with a three-drug antiretroviral regimen for a duration of 28 days is recommended (33). The preferred regimen is emtricitabine/tenofovir disoproxil fumarate (200 mg/300 mg) plus either raltegravir or dolutegravir (33). It is noteworthy to mention that non-pregnant women of childbearing potential who are sexually active or have been sexually assaulted and who are not using an effective birth control method, and pregnant women early in pregnancy should avoid dolutegravir because of potential fetal harm from exposure (35). The preferred PEP regimen for these women is raltegravir, tenofovir, and emtricitabine (35). In addition, there is recent evidence showing that bictegravir/emtricitabine/tenofovir alafenamide (Biktarvy®) co-formulated as a single daily pill is safe, well-tolerated and highly acceptable when used for PEP and it is commonly prescribed, even if it is not yet formally approved for PEP by Health Canada or the FDA in the U.S. (36–38).
The New York State Department of Health AIDS Institute Clinical Guidelines state that PEP should be initiated within two hours (“ideal”, because the effectiveness of PEP decreases over time after two hours) and no later than 72 hours after an exposure (5). According to the British Association for Sexual Health and HIV (BASHH) and British HIV Association (BHIVA) 2021 guideline, PEP should be initiated as soon as possible after exposure, preferably within 24 hours, but not beyond 72 hours after exposure (8, 9). Similar recommendations (i.e., as soon as possible following exposure and within 72 hours of exposure) are provided by Australian federal and territorial authorities (6, 7). The British Columbia Centre for Excellence in HIV/AIDS recommends starting PEP within two hours and no later than 72 hours after the potential exposure event (4). Alberta guidelines for post-exposure management and prophylaxis state that ideally PEP should be started within one to four hours of the exposure, and no longer than 72 hours (10). Belgian guidelines for non-occupational HIV PEP recommends PEP initiation as soon as possible, preferably within 24 hours of exposure but can be offered up to 72 hours (11). According to the European AIDS Clinical Society (EACS) 2022 guidelines, PEP should be started ideally <4 hours after the exposure, and no later than 48/72 hours (12). The Canadian guideline developed in 2017 recommends beginning PEP as soon as possible after an exposure, up to a maximum of 72 hours afterward (27). It is important to note that from a public health perspective, PEP complements but does not replace other HIV prevention methods such as condoms or PrEP. Some studies have reported that men who have sex with men seeking PEP were more likely to have subsequent HIV seroconversion and their risk increased stepwise with the number of PEP courses (13). For example, according to the national surveillance data from the UK, men who have sex with men who are prescribed PEP have a 2.5- to 5-fold increase in HIV acquisition in the months after PEP compared with men who have sex with men not requiring PEP (39). All these factors indicate the necessity of combining PEP with other HIV precautions, such as behavioural interventions, psychosocial support, drug use intervention, and PrEP (13, 40).
PEP awareness and uptake
A systematic review published in 2022 assessed the awareness and use of PEP among men who have sex with men worldwide (41). Overall, 20 eligible studies published between 2007 and 2021 were included in the meta-analysis, 13 of them from high-income countries, involving 12,579 men who have sex with men (41). The pooled estimate of the proportion of men who have sex with men who were aware of PEP was modest at 59.9%, and the proportion of those who previously used PEP was at 4.9% (41).
Another systematic review on PEP among men who have sex with men identified 74 studies (71 of them in high-income countries) (13). The pooled rate of PEP awareness and uptake was 51.6% and 6.0%, respectively (13). Pooled completion rate of PEP was 86.9% (13).
One analysis utilizing over three years of clinic information (2015–2018) from the Sexual Assault and Partner Abuse Care Program (SAPACP) at The Ottawa Hospital shows that of 1,032 sexual assault cases (90% female), 494 were eligible for PEP, and 307 of them (62%) initiated it (42). Among the key groups most likely to decline PEP were female patients with known assailants (42).
In 2019, a large study of a web-based sample of cisgender and transgender men who have sex with men (n=63,015) in the U.S. found that prior PEP use was reported by 11.28% (7,108 of 63,015) of the participants (43). Nearly half (3,268 of 7,108; 46%) of the past PEP users were current PrEP users, and another 39.9% (2,836 of 7,108) of the participants who reported past PEP use also reported prior PrEP use (43). Compared with White men, Black, Latine and those identifying as another race or ethnicity had higher prevalence of past PEP use (43). The study also found that men who have sex with men aged <25 years had lower prevalence of past PEP use than those aged 25 to 44 years, but this was expected in lifetime use statistics, given that older people have had more time to access PEP in the past (43).
Similar results were obtained by a survey of key at-risk populations conducted in 2016–2017 in New York City (44). Although 59.2% of respondents (n=313) were aware of PEP, there were significant differences by key population: PEP awareness was 80.2% among young men of color who have sex with men, 62.6% among trans women, and 33.5% among cisgender women of color (44). Overall, 13% of survey participants had ever used PEP (44).
Drug use seems to be significantly associated with PEP uptake: a study from New York found that compared with those who had not used methamphetamine in the last six months, young men who have sex with men who did use methamphetamine were six times more likely to have ever used PEP (adjusted odds ratio [AOR] = 6.07, 95% confidence interval [CI] 2.10–16.86) (45), and young men who have sex with men who had ever used PrEP had 16 times higher odds of ever using PEP (AOR = 16, 95% CI: 7.41–35.95) (45).
An Australian study of 232 men who have sex with men indicated that between two observation time periods (2015–2017 and 2018–2020), the number of men accessing PEP decreased significantly from 302 of 4,779 (6.3%) of visits to 221 of 7,205 (3.1%), when PrEP became more accessible through the Australian Pharmaceutical Benefits Scheme (46). On the other hand, PrEP uptake after presenting for PEP increased from 30 (12.9%) of total visits to 69 (34.2%) between the same time periods (46). While there is limited published research exploring relationships between PEP and PrEP use, men who have sex with men presenting at a health service requesting PEP are very likely to meet the guideline recommendations for PrEP (46). Research suggests that assessment of ongoing risk of HIV exposure and consideration of transfer to PrEP on completion of a course of PEP should be part of the overall management of PEP and be included in clinical policies and procedures (46, 47).
Research shows that the lockdowns in response to the COVID-19 pandemic had negative impact on PEP utilization. For example, data from six English sexual health clinics during COVID-19 show that the lockdowns had a negative impact on PEP dispensing numbers (48). In 2020, 2,884 PEP prescriptions were dispensed across the six centres studied, a fall of 34.5% from the 4,403 PEP prescriptions in 2019 (48). Before the COVID-related lockdown in 2020, the number of PEP prescriptions dispensed was stable at 82.5 per week (48). Following the first lockdown, this fell to a nadir of 13 (48). Prescriptions rose to a peak of 79 and then declined to 32 prescriptions in the last week of 2020 (48). There was no difference in the following characteristics of PEP recipients before and during the first lockdown: age, ethnicity, country of birth, or the service the recipient attended (48). London’s 56 Dean Street sexual health clinic in the UK observed similar trends during the lockdown: compared with the four-week period before lockdown (March 23, 2020), there were more than 80% fewer PEP prescriptions in the first four weeks of the lockdown (April 19, 2020): 161 vs 28 prescriptions (49). Although the most obvious explanation for this decline in PEP prescriptions is that individuals may have been engaging in less condomless sex during lockdown, people’s reluctance to travel during lockdowns could have at least partially contributed to decreased PEP utilization (48, 49). In addition to patients’ awareness and uptake of PEP, the literature also provides some insight into health care providers’ attitude and practices regarding PEP prescription. For example, a study of 153 emergency department physicians across seven emergency departments in Virginia found that although 91% and 87% of them were willing to prescribe PEP for intravenous drug use and unprotected sex, respectively, only 40% could confidently prescribe the appropriate regimen, and only 25% prescribed PEP in the last year (22). Participating health care providers considered time (27%), connecting patients to follow-up (26%), and cost to patients (23%), as barriers to prescribing PEP (22). In another study, HIV providers and non-HIV providers (n=480) practicing within above-average HIV prevalence ZIP codes of the ten U.S. cities with greatest overall HIV prevalence participated in a cross-sectional survey between 2014 and 2015 (23). Overall, 12.5% were unaware of PEP, 43.5% were aware but had not prescribed PEP, and only 44% had prescribed PEP for potential sexual exposures to HIV (23). The authors suggested that although PEP awareness was high in the sample, interventions were needed to support PEP prescription practices, optimize clinical protocols for PEP prescribing, and publicize PEP availability to the patients particularly in areas with lower levels of PrEP uptake (23). Similarly, an online survey of 820 primary care providers from six Southeastern U.S. cities in 2017 reported that 31% of them had “ever prescribed” PEP (24). In this study, prescribing PEP was strongly associated with PEP familiarity and prescribing PrEP (24).
PEP adherence and completion rates
PEP adherence and completion rates vary greatly depending on study jurisdictions and population groups receiving it. At the same time, there seems to be some contradictory evidence regarding the difference in completion rates comparing protease inhibitor (older) or integrase inhibitor/non-protease inhibitor (newer) regimens: a 2014 systematic review suggested no such difference (50), whereas a 2021 network meta-analysis of randomized-controlled trials (51) and some other studies (52) concluded that modern regimens with integrase inhibitors had better (i.e., higher) rates in PEP completion up to 90–92% (13, 53). The latest systematic review summarizing evidence from 2017–2022 found that newer PEP regimens containing TAF/FTC or TDF/FTC backbones and raltegravir, elvitegravir/cobicistat, dolutegravir, bictegravir (RAL, RPV, ELV/c, DTG, BIC), and long-acting intravenous albuvirtide are associated with high completion rates and minimal side effects (34).
According to a 2014 meta-analysis (n=21,462 PEP initiations), overall about 57% of people considered eligible for PEP completed the full standard 28-day course (50); however, this varied greatly among sub-groups of patients such as 67% among men who have sex with men, 66% among other non-occupational exposure, 56% among occupational exposure, and 40% among people who experienced sexual assault (50).
According to 2000–2014 data based on 3,547 PEP consults in Montreal, 70% of patients were adherent to PEP (54). Patients were predominantly male (92%), men who have sex with men (83%) and sought PEP after anal intercourse (72%) (54). Patients were more likely to be adherent to tenofovir/emtracitabine-based regimens (currently a recommended regimen in Canada) which are known to have better tolerability than older zidovudine/lamivudine-based regimens (not used in Canada anymore) (54). Similarly, data from the French Dat’AIDS cohort reporting on 19,240 PEP prescriptions between 2004 and 2017 show that 20% of PEP prescriptions were prematurely discontinued (55). Older age, men who have sex with men, intercourse with a sex worker, rape and intercourse with a known HIV-infected source patient were factors associated with increased rates of PEP completion (55). Follow-up HIV serological testing was completed only in 31% of participants (55).
Similarly, of the 282 courses of PEP dispensed at two community-based clinics in Los Angeles County (84% among men who have sex with men), only 47% were retained at week 24 (56). Unemployed individuals were less likely to be retained than employed individuals (56). At the week 2 and 4 visits, participants were asked to self-report their medication adherence: 53% of participants reported complete medication adherence, whereas 26% reported incomplete medication adherence, and 21% had unknown medication adherence due to missing the week 4 visit (56).
In a five-year prospective cohort from Brussels, Belgium, among 1,881 patients receiving PEP, 66.4% had a documented completion of a 28-day course of PEP (57). Adherence to PEP was higher among men who have sex with men, older patients, native Belgians, patients having a health insurance and previous PEP users (57).
People who experienced sexual assault (often reported in the literature as victims of sexual assault) represent a significant proportion of PEP recipients. As noted above, PEP adherence seems to be much lower among this population group. The numbers vary across the studies: an earlier 2014 meta-analysis (includes mostly high-income countries), PEP completion rates among people who experienced sexual assault (both female and male) were around 40% (50). According to a more recent (2018) meta-analysis of U.S. studies, the average percentage of people who experienced sexual assault (both female and male) who completed their PEP course ranged from 21.2% to 29%, with a mean of 25.7% (14). A recent study conducted at two U.S. emergency departments found similar rates: the prevalence of women who experienced sexual assault and completed a full course of PEP was 22.8% (n = 56), whereas 77.2% (n = 190) did not complete the full course (15). Factors that showed significant associations with sexually assaulted female patients completing PEP course included educational level, employment, health insurance, vaginal injuries, and tongue–mouth assaults (15). The above mentioned five-year Belgian data also showed that sexual assault survivors (90.8% of whom were female) demonstrated reduced adherence to PEP and were overexposed to deleterious effects of the absence of a health insurance in terms of compliance (57).
In addition to a lower reported rate of PEP completion among people who experienced sexual assault, there is a considerable lack of documented follow up. For example, a study from an emergency department in St. Louis, Missouri showed that out of 423 people who experienced sexual assault (95.5% female, 63.4% Black, 66.3% unemployed, 53.9% uninsured), completion of the full 28-day course of HIV PEP was documented in the electronic medical records for only 14 (3.3%) instances, 11 (3%) had documented non-completion and 343 (93.2%) lacked any documentation concerning adherence (16). Only 43 (11.7%) patients who accepted PEP and 1 (1.8%) patient who declined PEP received repeat HIV testing at six months following their initial emergency department visit (16). Studies from Europe show similar numbers. For example, data on 631 people who experienced sexual assault (93% women) prescribed PEP at an emergency department in Barcelona, Spain between 2006 and 2015 show that the follow-up rate was 38% at day 28, and the PEP completion rate at day 28 was 29% (17). Only 33% patients returned for HIV testing at day 90 (17).
Best practices of PEP delivery
PEP starter packs
According to the New York State Department of Health AIDS Institute Clinical Guidelines (PEP to Prevent HIV Infection, 2022), starter packs may reduce the time to PEP initiation and have been used in several PEP protocols, including emergency department visits following sexual assault (5). If a 28-day supply of medications cannot be provided, then in most cases, a seven-day supply will allow an individual sufficient time to access the additional medications needed to complete the full course of treatment (5). Patients who receive a seven-day starter pack should be informed that it does not contain the full 28-day course of PEP medication and assisted in creating a plan to obtain the rest of the required medications (5).
Similarly, in British Columbia, the Centre for Excellence for HIV/AIDS provides five-day starter kits of antiretroviral PEP in all emergency rooms in BC, outpost nursing stations, provincial prisons, and several Vancouver primary care and sexual health clinics (4). It is recommended that the five-day starter kit be initiated within two hours and no later than 72 hours after the potential exposure event, if at all possible (4).
According to a 2015 systematic review of 54 studies with data on 11,714 PEP initiations (21), PEP completion outcomes were better when participants were offered a full 28-day course of PEP at initial presentation to health care, with fewer refusals (11.4% vs 22%) and higher completion rates (70% vs 53.2%) (21). More than a quarter (28%) of individuals provided with a PEP starter pack failed to return for their subsequent appointment (21). The authors concluded that starter packs do not improve adherence to PEP and may result in lower adherence and completion rates (21). Providing starter packs (i.e., only a partial initial supply) enables prescribers to: reassess the need for PEP when baseline laboratory results become available, modify therapy in cases of drug intolerance or concerns about drug resistance, and limit drug costs and toxicities by preventing unnecessary use (27). However, based on the findings of the above-mentioned systematic review (21), the Canadian guideline recommends that when the indication for PEP is clearly established, the full course of PEP may be dispensed from the outset, rather than providing a starter pack (27). At the same time, the guideline authors acknowledge that this recommendation is weak; this is because variability in who (patients or the institutions that provide the starter packs) covers the cost of the medication in different contexts may lead to differences in which approach is favoured (27).
Standardization of PEP process
A U.S. study evaluated the association between the standardization of the PEP process in sexual assault patients process and medication errors in a large, academic health system (Cleveland Clinic Health System) with both freestanding and hospital-based emergency departments (EDs) (58). Pharmacists, doctors, nurses, and other stakeholders collaborated on a health system-wide standardization that included the development of an order set for STI testing and treatment, initial PEP dose administration in the ED, direct procurement of a complete 28-day PEP supply from an on-site specialty pharmacy, and follow-up with an infectious diseases physician scheduled before discharge (58). Data regarding the following medication errors were evaluated: incomplete regimen; inappropriate/duplicative regimen; dosing, frequency, or quantity prescribed error; and initiation of PEP without an HIV test (58). Among 206 patients, a higher proportion of patients experienced medication errors in the pre-standardization group relative to the post-standardization group (46.5% vs 11.9%) (58). There were 55 errors observed in the pre-standardization group, compared to 16 errors in the post-standardization group (58). The majority of errors in the pre-standardization group were directly related to antiretroviral regimens, while the majority of errors in the post-standardization group involved initiation of PEP without an HIV test (58). The authors concluded that optimization of medication-use technology and standardization of the PEP process, including clinical decision support in the electronic health records (EHR), was associated with lower odds of errors in ED patients (58).
Similar comprehensive PEP program consisting of a standardized order set, real-time multidisciplinary consultation, on-site pharmacy and close post-discharge follow-up was implemented between 2017 and 2018 at an ED in Arkansas (59). This standardization intervention resulted in improved guideline compliance with more frequent and appropriate PEP administration (59). Another study from a large urban centre in Florida evaluated PEP protocol implementation designed to facilitate PEP access (60). The PEP protocol has been revised to include: CDC prescription algorithm, pre-printed prescriptions, a pre-printed letter of medical necessity (required to access medication assistance programs), a release of medical information form, handouts describing medication use and side-effects, a handout listing partnering community clinics for follow-up care, and a letter for use if patients plan to follow-up with their own primary care provider (60). Chart review of 157 patients presented during the study period showed that mean time to care was 32.4 hours, with 126 (80%) presenting ≤72 hours; 114 (73%) patients were offered PEP by providers; 67 of these 114 (59%) patients accepted; the most common reason for declining was needing more time to decide (60). Overall, 83 of 99 (84%) patients clearly eligible by chart review were offered PEP (60).
Post-exposure prophylaxis in-pocket (PIP) and “self-start home PEPSE”
PIP intervention provides selected patients with a 28-day prescription for PEP before an exposure occurs, and they are counselled to obtain the medications and keep them accessible in case of an exposure. PIP is offered to those who report a low frequency (0-4 per year) of high-risk HIV exposures of any type (18, 19). Should there be an exposure, patients are advised to initiate medications as soon as possible (and within a 72-hour window) and to go to the clinic within the first week of initiating medications for clinical assessment and baseline HIV screening (61). Patients are typically followed at 6-month intervals for routine screening for HIV and STIs, or sooner based on their exposure history (61). The evidence about PIP is presented in two peer-reviewed articles and two 2023 conference abstracts based on research at the Toronto General Hospital HIV Prevention Clinic and St. Michael’s Hospital HIV Clinic (18, 62, 63). One of these publications reports on 30 patients prescribed PIP between 2013–2017, four (13.3%) of which actually used their medication (63), and the other publication reports on 79 patients prescribed PIP between 2016–2019, out of which 21 (26.6%) patients initiated their PIP medications, with a total of 32 PIP courses taken (62). Both of these studies mainly included men who have sex with men (with the exception of two women) (62, 63). The authors consider PIP a helpful HIV prevention modality for individuals with a low frequency of high-risk HIV exposures (61, 62). It enables immediate access to antiretroviral medications and might reduce the need for time-sensitive emergency department or clinic visits (61). In March 2023, updated 2016-2022 data (approximately 178 person-years) were shared from 111 people who primarily were men who have sex with men (94%) (18). A total of 69 courses of PIP during the observation period were self-initiated by 35 of the 111 people prescribed PEP (18). During the years of observation, patients transitioned between HIV prevention modalities as circumstances warranted: 33 (29%) changed from PIP to PrEP, and 35 individuals (31%) changed from PrEP to PIP (18). No HIV seroconversions have been reported throughout the duration of PIP intervention in these clinics (18). In April 2023, the latest data were shared at the Canadian Conference on HIV/AIDS Research (CAHR): among 112 patients aged 20–69 for a total follow-up of 183.8 patient-years, 18 episodes of bacterial STIs were diagnosed in 13 individuals (12%), but no HIV seroconversions have been reported (19).
An editorial comment by Wood (2020) concludes that PIP appears to be a reasonable option for certain men who have sex with men with low-frequency, high-risk exposures, especially if they tend not to anticipate sexual encounters (64). At the same time, Wood points out that there may be pitfalls to this strategy. For example, the protocol still requires a visit in clinic after initiation of at-home PEP and that even though adherence in the study was remarkably high, this requirement may be a hurdle for some individuals (64). In addition, diversion or misuse of the prescribed antiretrovirals may occur (64). Overall, Wood considers that, if replicated for other at-risk demographic groups and in larger, prospective trials, dissemination of PIP could help to individualize biomedical HIV prevention strategies and boost the number of individuals accessing them (64), and future investigations could examine complementary approaches to further reduce barriers to PIP, such as telemedicine or other electronic health approaches that eliminate the requirement for any clinic visit (64). A randomized-controlled trial from the UK investigated a similar (but not identical) approach – the role of advance provision of home packs of post-exposure prophylaxis for sexual exposure (so-called “self-start HOME PEPSE”) (20). In the UK, post-exposure prophylaxis after sexual exposure to HIV is often abbreviated as PEPSE (instead of PEP commonly used in North America). HOME PEPSE comprised a five-day pack of emtricitabine/tenofovir disoproxil fumarate and maraviroc 600 mg, given in advance and initiated following potential exposure to HIV (20). In total, 139 men who have sex with men were randomised 1:1; 69 to immediate HOME PEPSE and 70 to deferred HOME PEPSE (20). Of these, 31 in HOME PEPSE and 15 in deferred PEPSE arm initiated PEPSE (20). Uptake of HOME PEPSE was appropriate in 27 of 31 cases (87%) (20). Median time from exposure to first dose was 7.3 hours (range 3.0–20.9 hours) in the HOME PEPSE group, and 28.5 hours (range 17.3–34.0 hours) in the deferred HOME PEPSE group (20). HOME PEPSE was well tolerated with no discontinuations, and there were no significant differences in missed opportunities for PEPSE uptake, sexual behaviour or bacterial STIs between treatment arms (20). The authors concluded that self-start HOME PEPSE was safe to take and reduced the time from potential exposure to HIV to first PEP dose, from 29 hours to 7 hours (i.e., by 21 hours) (20). For comparison, in the UK, the average time to first PEP dose is 24 hours (20). This may have a significant benefit for effectiveness. The authors suggested that self-start HOME PEPSE packs could be included as part of the toolbox of HIV prevention (20).
Other examples of PEP delivery to various at-risk population groups described in literature include:
- Text-message support to prompt PEP patients to complete their course of medication and follow-up blood work, and to attend follow-up appointments, at St. Michael’s Hospital and Toronto General Hospital (65, 66)
- Nurse-led PEP follow-up to shift PEP delivery to sexual-health clinics and nurses, as a more accessible options for patients, at St. Michael’s Hospital and Toronto General Hospital (65, 66)
- Providing PrEP/PEP at a substance use disorder clinic located in an area experiencing an HIV outbreak among people who use drugs in Boston (67)
- PEP training program and a computer-based decision program using simulated patients in ED to improve quality and accuracy of PEP prescription at an academic hospital in Paris, France (68)
- The IN-STEP (Integrating PEP after Sexual Trauma in Emergency Practice) project designed to improve access to PEP after sexual assault at an emergency department of an academic hospital in New Mexico (69)
- Offering PrEP immediately after PEP (nurse-led PEP2PrEP) at an STI clinic in Ottawa (40, 70), resulting in decrease in seroconversion rate from 1.7% pre-PEP2PrEP to 0% post-PEP2PrEP per year (40)
- A simulation-based training on triage rules for triage nurses at an academic ED in Paris, France, with triage based on time between HIV-exposure and ED arrival, and providing five-day supply and an appointment at infectious diseases unit within 48–72 hours (71)
- HIV leadership program attendance (MpowermentYVR and Totally Outright) to increase PEP (and PrEP) awareness among young (<35 years) gay, bisexual, and other men who have sex with men in Vancouver (72)
- A simulation course and the Provider Sexual Assault Checklist (P-SAC) for emergency nurses to improve the timeliness of administering nonoccupational PEP to people who experienced sexual assault, implemented at an urban academic trauma center in Boston (73).
Appendix 1
Ontario guidelines for providers offering HIV testing. Algorithm for evaluation of possible post-exposure prophylaxis (PEP) treatment for high-risk non-occupational HIV exposures. 2023. (29).
Factors that may impact local applicability
There are several different regimens of antiretroviral medications used for PEP. Financial coverage for non-occupational PEP (e.g. after sexual exposure or drug use) by public health systems varies widely across different jurisdictions, including Canadian provinces and territories. Real world effectiveness of PEP is difficult to assess as there is limited follow-up or adherence monitoring after PEP provision in EDs. PEP provision strategies after possible HIV exposure and recommended PEP initiation timeframes differ across jurisdictions.
What we did
We searched Medline (including Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE®) using a combination of terms PEP or postexposure prophylaxis or post-exposure prophylaxis) in titles or abstracts AND HIV in titles or abstracts. Searches were conducted on February 27, 2023 and results limited to English articles published from 2018 to present. Studies from low- and middle-income countries were excluded. Reference lists of identified articles were also searched. Google (grey literature) searches using different combinations of these terms were also conducted. The searches yielded 618 references from which 73 were included.
Reference list
- Siedner MJ, Tumarkin E, Bogoch, II. HIV post-exposure prophylaxis (PEP). BMJ. 2018;363:k4928.
- Centers for Disease Control and Prevention. What is PEP? Available from: https://www.cdc.gov/hiv/basics/pep/about-pep.html Accessed March 22, 2023.
- HIVinfo.NIH.gov. HIV prevention. Post-exposure prophylaxis (PEP). 2021. Available from: https://hivinfo.nih.gov/understanding-hiv/fact-sheets/post-exposure-prophylaxis-pep Accessed October 5, 2023.
- British Columbia Centre for Excellence in HIV/AIDS. Guidance for the use of post-exposure prophylaxis (PEP) for the prevention of HIV in British Columbia. 2020. Available from: https://bccfe.ca/sites/default/files/uploads/publications/centredocs/guidance_for_the_use_of_post-exposure_prophylaxis_pep_for_the_prevention_of_hiv_in_british_columbia_31march202.pdf Accessed March 21, 2023.
- DeHaan E, McGowan JP, Fine SM, Vail R, Merrick ST, Radix A, et al. New York State Department of Health AIDS Institute Clinical Guidelines 2022. PEP to prevent HIV infection. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562734/pdf/Bookshelf_NBK562734.pdf Accessed March 20, 2023.
- Australasian Society for HIV Viral Hepatitis and Sexual Health Medicine (ASHM). National guidelines for post-exposure prophylaxis after non-occupational and occupational exposure to HIV (Second edition). 2016. Available from: https://ashm.org.au/wp-content/uploads/2022/04/PEP_GUIDELINES_2016.FINAL_ONLINE_VERSION-1.pdf Accessed March 21, 2023.
- Government of Western Australia, Department of Health. Guideline for non-occupational post-exposure prophylaxis (NPEP) to prevent HIV in Western Australia. 2021. Available from: https://www.health.wa.gov.au/~/media/Corp/Documents/Health-for/Communicable-Diseases/Guidelines/Guideline-for-NPEP-in-WA.pdf Accesed March 21, 2022.
- Cresswell F, Asanati K, Bhagani S, Boffito M, Delpech V, Ellis J, et al. UK guideline for the use of HIV post-exposure prophylaxis 2021. HIV Medicine. 2022;23(5):494–545.
- British HIV Association (BHIVA). UK guideline for the use of HIV post-exposure prophylaxis 2021 (post consultation version). Available from: https://www.bhiva.org/file/6183b6aa93a4e/PEP-guidelines.pdf Accessed March 21, 2023.
- Ministry of Health, Government of Alberta. Alberta guidelines for post-exposure management and prophylaxis: HIV, hepatitis B, hepatitis C and sexually transmitted infections. 2019. Available from: https://open.alberta.ca/publications/9781460143360 Accessed April 24, 2023.
- Libois A, Florence E, Derdelinckx I, Yombi JC, Henrard S, Uurlings F, et al. Belgian guidelines for non-occupational HIV post-exposure prophylaxis 2017. Acta Clinica Belgica. 2018;73(4):275-80.
- EACS European AIDS Clinical Society. Guidelines version 11.1 October 2022. Available from: https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf Accessed May 29,2023.
- Wang Z, Yuan T, Fan S, Qian HZ, Li P, Zhan Y, et al. HIV nonoccupational postexposure prophylaxis among men who have sex with men: A systematic review and meta-analysis of global data. AIDS Patient Care & STDs. 2020;34(5):193–204.
- Scannell M, Kim T, Guthrie BJ. A meta-analysis of HIV postexposure prophylaxis among sexually assaulted patients in the United States. Journal of the Association of Nurses in AIDS Care. 2018;29(1):60–9.
- Scannell MJ, Rodgers RF, Molnar BE, Guthrie BJ. Factors impacting HIV postexposure prophylaxis among sexually assaulted patients presenting to two urban emergency departments. Journal of Forensic Nursing. 2022;18(4):204–13.
- Cherabie JN, Gleason E, Munigala S, Fox B, Trolard A, McCammon C, et al. Post-exposure prophylaxis for human immunodeficiency virus after sexual assault in a Midwestern U.S. emergency department. American Journal of Emergency Medicine. 2021;49:117–23.
- Inciarte A, Leal L, Masfarre L, Gonzalez E, Diaz-Brito V, Lucero C, et al. Post-exposure prophylaxis for HIV infection in sexual assault victims. HIV Medicine. 2020;21(1):43–52.
- Billick MF, KN, Myers S, Tan D, Bogoch II. PEP-in-Pocket (PIP): Long-term follow-up of on demand HIV post-exposure prophylaxis. Conference on Retroviruses and Opportunistic Infections (CROI) 2023. Available from: https://www.croiconference.org/wp-content/uploads/sites/2/posters/2023/CROI_2023_PIP_Poster_FINAL_ONLINE-133207847422481529.pdf Accessed March 28, 2023.
- Billick M, Fisher K, Myers S, Tan D, Bogoch I. PEP-in-Pocket (PIP): Long-term follow-up of on demand HIV post-exposure prophylaxis. The 32d Annual Canadian Conference on HIV/AIDS Research (CAHR). 2023. Available from: https://www.cahr-acrv.ca/wp-content/uploads/2023/04/CAHR2023-Abstract-Book_as-of-April-24.pdf Accessed May 26, 2023.
- Fox JM, Lee MJ, Fairhead CL, Ledwaba-Chapman LM, Nori AV, McQuillan O, et al. Self-start HIV postexposure prophylaxis (PEPSE), to reduce time to first dose and increase efficacy. Sexually Transmitted Infections. 2022;23:23.
- Ford N, Venter F, Irvine C, Beanland RL, Shubber Z. Starter packs versus full prescription of antiretroviral drugs for postexposure prophylaxis: A systematic review. Clinical Infectious Diseases. 2015;60(suppl_3):S182–S6.
- O’Connell KA, Kisteneff AV, Gill SS, Edwards JF, Sherrerd-Smith WW, Moraczewski LA, et al. HIV post-exposure prophylaxis in the emergency department: An updated assessment and opportunities for HIV prevention identified. American Journal of Emergency Medicine. 2021;46:323–8.
- John SA, Quinn KG, Pleuhs B, Walsh JL, Petroll AE. HIV post-exposure prophylaxis (PEP) awareness and non-occupational PEP (nPEP) prescribing history among U.S. healthcare providers. AIDS & Behavior. 2020;24(11):3124–31.
- Henny KD, Duke CC, Buchacz K, Brooks JT, Samandari T, Sutton MY. HIV prescriptions on the frontlines: Primary care providers’ use of antiretrovirals for prevention in the Southeast United States, 2017. Preventive Medicine. 2020;130:105875.
- CATIE. Post-exposure prophylaxis (PEP). 2019. Available from: https://www.catie.ca/post-exposure-prophylaxis-pep Accessed March 24, 2023.
- Heendeniya A, Bogoch, II. Antiretroviral medications for the prevention of HIV infection: A clinical approach to preexposure prophylaxis, postexposure prophylaxis, and treatment as prevention. Infectious Disease Clinics of North America. 2019;33(3):629–46.
- Tan DHS, Hull MW, Yoong D, Tremblay C, O’Byrne P, Thomas R, et al. Canadian guideline on HIV pre-exposure prophylaxis and nonoccupational postexposure prophylaxis. Canadian Medical Association Journal. 2017;189(47):E1448–E58.
- Public Health Agency of Canada. HIV factsheet: Biomedical prevention of HIV—PrEP and PEP. 2021. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/hiv-factsheet-biomedical-prevention-prep-pep.html Accessed March 22, 2023.
- Ontario guidelines for providers offering HIV testing. Algorithm for evaluation of possible post-exposure prophylaxis (PEP) treatment for high-risk non-occupational HIV exposures. 2023. Available from: https://hivtestingontario.ca/wp-content/uploads/2023/03/Ontario-HIV-Testing-Guidelines-for-Providers.pdf#page=12 Accessed May 26, 2023.
- Ontario Drug Benefit Formulary/Comparative Drug Index. DIN 02478579 Biktarvy 50mg & 200mg & 25mg Tab. Available from: https://www.formulary.health.gov.on.ca/formulary/results.xhtml?q=Biktarvy&type=2 Accessed May 26, 2023.
- Yoong D, St. Michael’s Hospital Toronto ON. Provincial/territorial coverage of ARV drugs for HIV prevention across Canada: Post-exposure prophylaxis (PEP) and pre-exposure prophylaxis (PrEP). 2018. Available from: https://hivclinic.ca/wp-content/uploads/2018/04/ARV-Coverage_PEP-and-PrEP.pdf Accessed April 24, 2023.
- The Ontario HIV Treatment Network (OHTN), Rapid Response Service. The efficacy of post-exposure prophylaxis (PEP) for HIV. 2019. Available from: https://www.ohtn.on.ca/rapid-response-the-efficacy-of-post-exposure-prophylaxis-pep-for-hiv/ Accessed April 25, 2023.
- Centers for Disease Control and Prevention. Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV—United States, 2016. Available from: https://stacks.cdc.gov/view/cdc/38856 Accessed March 20,2023.
- Tu J, Habib M, O’Byrne P, Cox J, Arkell C, Shukalek C, et al. Updating Canadian guidelines on HIV post-exposure prophylaxis: A systematic review of clinical trials & cohort studies. The 32d Annual Canadian Conference on HIV/AIDS Research (CAHR). 2023. Available from: https://www.cahr-acrv.ca/wp-content/uploads/2023/04/CAHR2023-Abstract-Book_as-of-April-24.pdf Accessed May 26, 2023.
- Centers for Disease Control and Prevention. Interim statement regarding potential fetal harm from exposure to dolutegravir—Implications for HIV post-exposure prophylaxis (PEP). Available from: https://stacks.cdc.gov/view/cdc/38856/cdc_38856_DS2.pdf Accessed March 20, 2023.
- Tan D, Persaud R, Qamar A, Bogoch I, Chan A, Chris A, et al. High tolerability and adherence with bictegravir, emtricitabine and tenofovir alafenamide as HIV post-exposure prophylaxis. The 32d Annual Canadian Conference on HIV/AIDS Research (CAHR). 2023. Available from: https://www.cahr-acrv.ca/wp-content/uploads/2023/04/CAHR2023-Abstract-Book_as-of-April-24.pdf Accessed May 26, 2023.
- Mayer KH, Gelman M, Holmes J, Kraft J, Melbourne K, Mimiaga MJ. Safety and tolerability of once daily coformulated bictegravir, emtricitabine, and tenofovir alafenamide for postexposure prophylaxis after sexual exposure Journal of Acquired Immune Deficiency Syndromes. 2022;90(1):27–32.
- Gilead Sciences Inc. Biktarvy® (BIC/FTC/TAF) use for HIV-1 post-exposure prophylaxis. 2023. Available from: https://www.askgileadmedical.com/docs/biktarvy/biktarvy-post-exposure-prophylaxis Accessed May 26, 2023.
- Mitchell H, Furegato M, Hughes G, Field N, Nardone A. What are the characteristics of, and clinical outcomes in men who have sex with men prescribed HIV postexposure prophylaxis following sexual exposure (PEPSE) at sexual health clinics in England? Sexually Transmitted Infections. 2017;93(3):207–13.
- O’Byrne P, Orser L, Vandyk A. Immediate PrEP after PEP: Results from an observational nurse-led PEP2PrEP study. Journal of the International Association of Providers of AIDS Care. 2020;19:2325958220939763.
- Jin J, Sun R, Mu T, Jiang T, Dai L, Lu H, et al. Awareness and use of post-exposure prophylaxis for HIV prevention among men who have sex with men: A systematic review and meta-analysis. Frontiers in Medicine. 2021;8:783626.
- Shipeolu L, Sampsel K, Reeves A, Blaskovits F, Heimerl M, Muldoon K. HIV nonoccupational postexposure prophylaxis for sexual assault cases: A 3-year investigation. AIDS. 2020;34(6):869–76.
- John SA, Sizemore KM, Jimenez RH, Jones SS, Petroll AE, Rendina HJ. The use of HIV pre- and postexposure prophylaxis among a web-based sample of HIV-negative and unknown status cisgender and transgender sexual minority men: Cross-sectional study. JMIR Public Health and Surveillance. 2022;8(12):e31237.
- Koblin BA, Usher D, Nandi V, Tieu HV, Bravo E, Lucy D, et al. Post-exposure prophylaxis awareness, knowledge, access and use among three populations in New York City, 2016–17. AIDS & Behavior. 2018;22(8):2718–32.
- Kaplun E, Martino RJ, Krause KD, Briganti M, D’Avanzo PA, Halkitis PN. Post-exposure prophylaxis and methamphetamine use among young sexual minority men: The P18 cohort study. International Journal of Environmental Research & Public Health. 2022;19(2):09.
- Woodward SC, Baynes AM, Tyson HA, Dunlop WA, Martin SJ. Is non-occupational HIV post exposure prophylaxis (nPEP) still used? An exploration of nPEP use since widespread availability of HIV PrEP. International Journal of STD & AIDS. 2022;33(10):914–9.
- Jain S, Krakower DS, Mayer KH. The transition from postexposure prophylaxis to preexposure prophylaxis: An emerging opportunity for biobehavioral HIV prevention. Clinical Infectious Diseases. 2015;60(Suppl 3):S200–S4.
- Whitlock GG, Ahmed N, Nori A, Richardson D, Clarke E, Browne R, et al. Characteristics of HIV post-exposure prophylaxis recipients at six English sexual health clinics during COVID-19. HIV Medicine. 2022;23(10):1103–7.
- Junejo M, Girometti N, McOwan A, Whitlock G. HIV postexposure prophylaxis during COVID-19. The Lancet HIV. 2020;7(7):e460.
- Ford N, Irvine C, Shubber Z, Baggaley R, Beanland R, Vitoria M, et al. Adherence to HIV postexposure prophylaxis: A systematic review and meta-analysis. AIDS. 2014;28(18):2721–7.
- Fernandez I, de Lazzari E, Inciarte A, Diaz-Brito V, Milinkovic A, Arenas-Pinto A, et al. Network meta-analysis of post-exposure prophylaxis randomized clinical trials. HIV Medicine. 2021;22(3):218–24.
- Malinverni S, Bedoret F, Bartiaux M, Gilles C, De Wit S, Libois A. Single-tablet regimen of emtricitabine/tenofovir disoproxil fumarate plus cobicistat-boosted elvitegravir increase adherence for HIV postexposure prophylaxis in sexual assault victims. Sexually Transmitted Infections. 2021;97(5):329–33.
- McAllister JW, Towns JM, McNulty A, Pierce AB, Foster R, Richardson R, et al. Dolutegravir with tenofovir disoproxil fumarate-emtricitabine as HIV postexposure prophylaxis in gay and bisexual men. AIDS. 2017;31(9):1291–5.
- Thomas R, Galanakis C, Vézina S, Longpré D, Boissonnault M, Huchet E, et al. Adherence to post-exposure prophylaxis (PEP) and incidence of HIV seroconversion in a major North American cohort. PLoS One. 2015;10(11):e0142534.
- Gantner P, Allavena C, Duvivier C, Cabie A, Reynes J, Makinson A, et al. Post-exposure prophylaxis completion and condom use in the context of potential sexual exposure to HIV. HIV Medicine. 2020;21(7):463–9.
- Beymer MR, Kofron RM, Tseng CH, Bolan RK, Flynn RP, Sayles JM, et al. Results from the post-exposure prophylaxis pilot program (P-QUAD) demonstration project in Los Angeles County. International Journal of STD & AIDS. 2018;29(6):557–62.
- Malinverni S, Gennotte AF, Schuster M, De Wit S, Mols P, Libois A. Adherence to HIV post-exposure prophylaxis: A multivariate regression analysis of a 5 years prospective cohort. Journal of Infection. 2018;76(1):78–85.
- Segovia MF, Pallotta AM, Campbell MJ, Englund K, Reali-Sorrell M, Shah SS. Impact of a standardized order set and medication-use process on medication error rates in sexual assault patients presenting to the emergency department for HIV nonoccupational postexposure prophylaxis. American Journal of Health System Pharmacy. 2022;14:14.
- Silva-Nash J, Bordelon S, Searcy SA, Dare RK. Standardizing HIV post-exposure prophylaxis in the emergency department following sexual assault. HIV Medicine. 2022;23(3):268–73.
- Ortega B, Thayer J, Chen L, Steblin S, Mhaskar RS, Straub DM. nPEP protocol implementation and evaluation at a local US Crisis Center. AIDS Care. 2022;34(10):1268–75.
- Heendeniya A, Bogoch, II. HIV prevention with post-exposure prophylaxis-in-pocket. The Lancet Public Health. 2019;4(10):e494.
- Alghamdi A, Hempel A, Heendeniya A, Clifford-Rashotte M, Tan DHS, Bogoch, II. HIV postexposure prophylaxis-in-pocket: Long-term follow-up of individuals with low-frequency, high-risk HIV exposures. AIDS. 2020;34(3):433–7.
- Tumarkin E, Heendeniya A, Murphy P, Placido T, Tan DHS, Bogoch, II. Brief report: HIV postexposure prophylaxis-in-pocket (“PIP”) for individuals with low-frequency, high-risk HIV exposures. Journal of Acquired Immune Deficiency Syndromes. 2018;78(1):20–2.
- Wood BR. Prevention of HIV for persons with low-frequency, high-risk exposures: PrEP (preexposure prophylaxis), PEP (postexposure prophylaxis), or ‘PIP’ (postexposure prophylaxis in-pocket). AIDS. 2020;34(3):481–2.
- MAP Centre for Urban Health Solutions, St. Michael’s Hospital, Toronto. Optimizing the delivery of HIV post-exposure prophylaxis: A randomized controlled trial of text messaging support and physician to nurse task-shifting (OPT-IN). Available from: https://maphealth.ca/opt-in/ Accessed May 26, 2023.
- ClinicalTrials.gov. Optimizing the delivery of HIV nPEP. Available from: https://clinicaltrials.gov/ct2/show/NCT03259698 Accessed May 26, 2023.
- Braun HM, Walter C, Farrell N, Biello KB, Taylor JL. HIV exposure prophylaxis delivery in a low-barrier substance use disorder bridge clinic during a local HIV outbreak at the onset of the COVID-19 pandemic. Journal of Addiction Medicine. 2022;16(6):678–83.
- Casalino E, Bouzid D, Antoniol S, Choquet C, Colosi L, Pereira L, et al. Assessment of HIV-postexposure prophylaxis prescription quality after a training programme and assistance in decisions provided by a computer-based decision program: A cross-over study. Acta Clinica Belgica. 2022;77(3):495–509.
- Saadatzadeh T, Salas NM, Walraven C, Sarangarm P, Crandall CS, Crook J, et al. Improving emergency access to human immunodeficiency virus prophylaxis for patients evaluated after sexual assault. Journal for Healthcare Quality. 2021;43(2):82–91.
- O’Byrne P, MacPherson P, Orser L. Nurse-led HIV PEP program used by men at high risk for HIV seroconversion. Journal of the Association of Nurses in AIDS Care. 2018;29(4):550-9.
- Casalino E, Kenway P, Bouzid D, Goncalves S, Antoniol S, Radou L, et al. Implementation of HIV-exposures triage strategy in emergency departments to improve nurse-triage for HIV-exposures: A pre- and post-intervention period study. International Emergency Nursing. 2019;47:100786.
- Closson K, Chown S, Armstrong HL, Wang L, Bacani N, Ho D, et al. HIV leadership programming attendance is associated with PrEP and PEP awareness among young, gay, bisexual, and other men who have sex with men in Vancouver, Canada. BMC Public Health. 2019;19(1):429.
- Scannell M, MacDonald AE, Berger A, Boyer N. The priority of administering HIV postexposure prophylaxis in cases of sexual assault in an emergency department. Journal of Emergency Nursing. 2018;44(2):117–22.e1.
Suggested citation
Rapid Response Service. Effectiveness, uptake and delivery of non-occupational HIV post-exposure prophylaxis (PEP). Toronto, ON: The Ontario HIV Treatment Network; October 2023.
Prepared by
David Gogolishvili and Rodney Kort
Illustration by
Rachel Chung